Benzethonium chloride benzethonium chloride
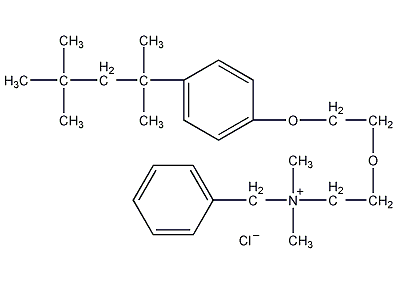

Structural formula
| Business number | 03DR |
|---|---|
| Molecular formula | C27H42ClNO2 |
| Molecular weight | 448.08 |
| label |
Quaternary ammonium salt 1622, N,N-dimethyl-N-[2-[2-4(1,1,3,3-tetramethylbutyl)phenoxy]ethyl]benzylamine chloride, Benzylethoxyamine chloride, Haiamine 1622, benzethonium chloride, Hamming 1622 solution, Anisinium chloride, Haiming1622, (2-(2-(4-diisobutylphenoxy)ethoxy)ethyl)dimethylbenzylammoniumchloride, 2-(2-(p-(diisobutyl), 2-(2-(p-(diisobutyl)phenoxy)ethoxy)ethyl, 2-(2-(p-(diisobutyl)phenoxy)ethoxy)ethyldimethylammoniumchloride, ammonium,benzyldimethyl(2-(2-(p-(1,1,3,3-tetramethylbutyl, Cationic surfactant |
Numbering system
CAS number:121-54-0
MDL number:MFCD00011742
EINECS number:204-479-9
RTECS number:BO7175000
BRN number:3898548
PubChem number:24886103
Physical property data
1. Properties: colorless hexagonal flake crystals
2. Melting point (℃): 164-166
3. PH value (1% aqueous solution): 4.8-5.5
4. Water solubility (g/100mL, 18 ºC): 1-5
Toxicological data
Acute toxicity:
Oral LD50 338mg/kg(mus)
368mg/kg(rbt)
Eye irritation severe 30ug(rbt)
p>
Main irritant effects:
On skin: Causes corrosive effects on skin and mucous membranes.
Irritation to skin and mucous membranes
On eyes: Strong corrosive effects
Irritation effects
Sensitization: None sensitizing effects of knowledge.
Ecological data
General remarks
Water hazard level 3 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow this product to come into contact with groundwater, waterways or sewage systems, even in small amounts.
Even extremely small amounts of product seeping into the ground can pose a hazard to drinking water.
Toxic to fish and plankton in the water
Do not discharge materials into the surrounding environment without government permission.
Highly toxic to organic matter in water.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 18.5
7. Number of heavy atoms: 31
8. Surface charge: 0
9. Complexity: 466
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Basic properties
Colorless hexagonal flaky crystals. Very bitter taste. Sensitive to light and air. Easily soluble in water, forming foam liquid, soluble in ethanol, acetone and chloroform. The pH of its 1% aqueous solution is 4.8 to 5.5. Slightly melts at 120℃. Melting point 164~166℃. Irritating.
Storage method
2. Storage:
Save in a dry and dark place.
Synthesis method
3. Brief description of the production method
Preparation of p-(1,1,3,3-tetramethyl from p-(diisobutyl)phenol, dichlorodiethyl ether and dimethylamine Butyl) phenoxyethoxyethyl dimethylamine, then warm it with benzyl chloride in benzene for 2 hours, evaporate the benzene, and obtain benzethonium chloride.
Purpose
4. Use
Cationic surfactant. A co-solvent that hydrolyzes the gel. Disinfectant and antiseptic.
