Benzyl alcohol Benzyl alcohol
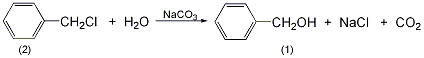
Structural formula
| Business number | 02J4 |
|---|---|
| Molecular formula | C7H8O |
| Molecular weight | 108.14 |
| label |
Benzyl alcohol, benzyl alcohol, α-Hydroxytoluene, Benzenemethanol, Phenylcarbinol, Phenylmethanol, high boiling point solvents, paint stripper, preservative, alcohol solvent |
Numbering system
CAS number:100-51-6
MDL number:MFCD00004599
EINECS number:202-859-9
RTECS number:DN3150000
BRN number:878307
PubChem number:24858412
Physical property data
1. Properties: Colorless and transparent liquid with a faint honey-sweet fruit aroma.
2. Boiling point (ºC, 101.3kPa): 205.45
3. Melting point (ºC): -15.3
4. Relative density (g/mL, 25/4ºC): 1.0456
5. Refractive index (n20DºC): 1.5400
6. Viscosity (mPa·s, 15ºC): 7.760
7. Viscosity (mPa·s, 30ºC): 4.650
8. Flash point (ºC, opening): 93
9. Fire point (ºC): 436.1
10. Heat of evaporation (KJ/mol, b.p.): 50.52
11. Heat of fusion (KJ/mol): 17.05
12. Heat of generation (KJ/mol): -161.1
13. Heat of combustion (KJ/mol): 3739.7
14. Specific heat capacity (KJ/(kg·K), 15~20ºC, constant pressure): 2.26
15. Conductivity (S/m, 25ºC): 18×10-7
16. Volume expansion coefficient (K-1 ): 0.00075
17. Vapor pressure (kPa, 58ºC): 0.13
18. Solubility (%, 20ºC, water): 3.8
19. Dissolution Properties: Slightly soluble in water, miscible with ethanol, ether, chloroform, etc. It can dissolve nitrocellulose, benzyl acetate, coumarone resin, glyceryl trirosinate, frankincense, casein, gelatin, shellac, etc.
20. Relative density (25℃, 4℃): 1.041
21. Refractive index at room temperature (n25): 1.5384
22. Critical temperature (ºC): 441.85
23. Critical pressure (MPa): 4.3
24. Eccentricity factor: 0.691
25. Solvent Degree parameter (J·cm-3)0.5: 24.649
26. van der Waals area (cm2· mol-1): 8.110×109
27. van der WaaObtained by heating and hydrolysis. 3.Mix benzyl chloride, sodium carbonate and water in a certain ratio, stir quickly to emulsify the oil layer, and raise the temperature to 90°C while stirring , when the generated carbon dioxide gas no longer escapes, continue to raise the temperature to 101~103℃ to complete the hydrolysis reaction: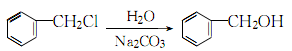 After the reaction is completed, cool and let stand for layering. Take the oil layer and wash it with water several times, merge it into the water layer, and then distill it under normal pressure. First, water, benzyl chloride and other low-boiling substances are evaporated as the initial fraction, and then the temperature between 203 and 206°C is collected as the main fraction, which is the finished product. 4. Tobacco: BU, 56; OR, 57; BU, 13; FC, 9, 18; BU, OR, 18; FC, BU, OR, 40. 5. Preparation method:
After the reaction is completed, cool and let stand for layering. Take the oil layer and wash it with water several times, merge it into the water layer, and then distill it under normal pressure. First, water, benzyl chloride and other low-boiling substances are evaporated as the initial fraction, and then the temperature between 203 and 206°C is collected as the main fraction, which is the finished product. 4. Tobacco: BU, 56; OR, 57; BU, 13; FC, 9, 18; BU, OR, 18; FC, BU, OR, 40. 5. Preparation method: 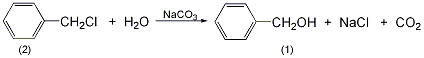 In a reaction bottle equipped with a stirrer, reflux condenser, and thermometer, add 250 mL of water, 56 g of sodium carbonate, and chloride 63.3g (0.5mol) of benzyl (2), stir vigorously to emulsify benzyl chloride. Add until slightly boiling (about 95°C). Carbon dioxide is produced during the reaction. When the carbon dioxide bubbles stop escaping, the reaction is completed, and then the reaction is stirred for 0.5 h. Cool, separate the organic layer, and wash with water to obtain crude benzyl alcohol. Fractionate distillate and collect the fractions at 204-205°C to obtain 40g of benzyl alcohol ①(1), with a yield of 74%. [1]
In a reaction bottle equipped with a stirrer, reflux condenser, and thermometer, add 250 mL of water, 56 g of sodium carbonate, and chloride 63.3g (0.5mol) of benzyl (2), stir vigorously to emulsify benzyl chloride. Add until slightly boiling (about 95°C). Carbon dioxide is produced during the reaction. When the carbon dioxide bubbles stop escaping, the reaction is completed, and then the reaction is stirred for 0.5 h. Cool, separate the organic layer, and wash with water to obtain crude benzyl alcohol. Fractionate distillate and collect the fractions at 204-205°C to obtain 40g of benzyl alcohol ①(1), with a yield of 74%. [1]
Purpose
1. Solvent for gelatin, casein (when hot), cellulose acetate, shellac, etc. Embedding materials for microscopy. Measure the content of vitamin B12. Organic Synthesis. It is a useful fragrance fixative and can be used as a raw material for the preparation of jasmine, moonseed, ylang-ylang and other essences. It is used in the preparation of soaps, daily cosmetic fragrances, and for medicinal and synthetic chemical industries. Product quality: medicinal content ≥98%, industrial content ≥95%. Used as high boiling point solvent, paint stripper, and preservative. Also used in the manufacture of plasticizers, spices, ballpoint pen oil, etc.
2.Benzyl alcohol is widely used in the production of industrial chemicals. Used in paint solvents, photographic developers, polyvinyl chloride stabilizers, pharmaceuticals, synthetic resin solvents, vitamin B injection solvents, and preservatives in ointments or liquids. It can be used as a drying agent for nylon filaments, fibers and plastic films, a solvent for dyes, cellulose esters and casein, and an intermediate for preparing benzyl esters or ethers. At the same time, it is widely used in pen making (ballpoint pen oil), paint solvents, etc. Benzyl alcohol is a very useful fixative and an indispensable spice in the preparation of jasmine, moonflower, ylang-ylang and other flavors. Used for preparing soaps and daily cosmetic essences. However, benzyl alcohol can slowly and naturally oxidize, and part of it generates benzaldehyde and benzyl ether, which makes commercially available products often have an almond aroma, so it should not be stored for a long time.
3.Can be used to prepare grape, cherry, berry, nut, sweet orange and other food flavors. Generally in chewing gum 1200mg/kg; in candy 47mg/kg; in baking 220mg/kg in baked goods; 160mg/kg in cold drinks; soft drinks 15mg/kg.
4.Used as solvent. Used as chromatography derivatization reagent and benzyl esterification reagent for organic acids. It is also used as an agent for organic synthesis and microscopic analysis.
5.In addition to being used to prepare flavors, it can also be used as an additive in medical injections, ointments or liquids. preservative. It is also used as a preservative in cosmetics, with a maximum allowable content of 1% in cosmetics. It can also be used as a desiccant for nylon filaments, fibers and plastic films, a solvent for dyes, cellulose esters and casein, and an intermediate for preparing benzyl esters or ethers. It is also widely used in pen making (ballpoint pen oil) and paint solvents. , ink additives, etc.
