Benzyl butyl phthalate Benzyl butyl phthalate
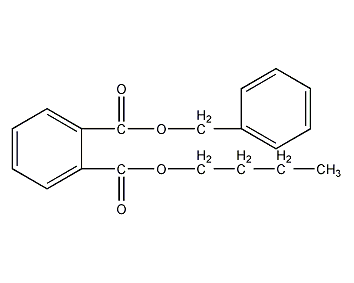
Structural formula
| Business number | 01W4 |
|---|---|
| Molecular formula | C19H20O4 |
| Molecular weight | 312.36 |
| label |
Butylbenzyl phthalate, Butylbenzyl phthalate, Plasticizer BBP, Butyl benzyl phthalate ester, Phthalic acid benzyl butyl ester, Plasticizer BBP, Plasticizer |
Numbering system
CAS number:85-68-7
MDL number:MFCD00009440
EINECS number:201-622-7
RTECS number:TH9990000
BRN number:2062204
PubChem number:24862813
Physical property data
1. Properties: Transparent oily liquid, flammable, with a weak odor.
2. Density (g/mL, 25/4℃): 1.113~1.121
3. Relative vapor density (g/mL, air=1): Undetermined
p>
4. Melting point (ºC): -35
5. Boiling point (ºC, normal pressure): 370
6. Boiling point (ºC, 5.2kPa): Not available Determine
7. Refractive index (25ºC): 1.5360
8. Flash point (ºC): 210
9. Viscosity (mPa·s, 20ºC) : 65
10. Autoignition point or ignition temperature (ºC): 240
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in organic solvents and hydrocarbons .
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 88.18
2. Molar volume (cm3/mol): 275.4
3. Isotonic specific volume (90.2K): 708.4
4. Surface tension (dyne/cm): 43.7
5. Polarizability (10-24cm3): 34.95
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 9
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 374
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13 .Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
It has good compatibility with most resins and has strong solvation effect.
Storage method
Seal and store in a dry and dark place at 4°C. It should be stored in a ventilated and dry place indoors to prevent moisture intrusion and keep the container closed. This product is flammable. Fire sources should be avoided and cannot be stored together with oxidants or explosive materials.
Synthesis method
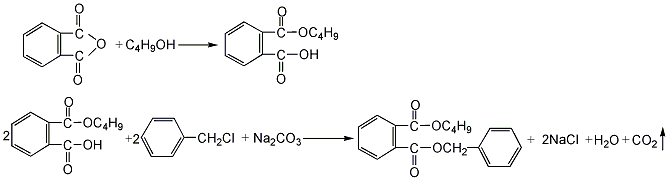
1. Phthalic anhydride and butanol are esterified in a mass ratio of 1:0.52 to first generate monobutyl phthalate. Then add 30% soda ash dropwise, the weight of which is approximately equal to the mass of phthalic anhydride, and then add benzyl chloride equal to the mass of phthalic anhydride for condensation reaction. After the condensation reaction is completed, water is added for washing, the crude ester is steam distilled, and further vacuum distilled to obtain the finished product.

2, Monobutyl phthalate is obtained by esterifying phthalic anhydride and butanol in a mass ratio of 1:0.52, and then performs a second esterification with benzyl chloride in the presence of sodium carbonate. It is obtained by neutralizing with alkali solution, washing with water and distilling under reduced pressure.
3. Equipped with electric stirring, thermometer and reflux condenser tube In a 250mL three-necked flask, add phthalic anhydride and n-butanol at a molar ratio of 1:1.05, stir at (110±2)°C for 2 hours, then cool to 60~80°C, and slowly add 30% NaOH aqueous solution dropwise Neutralize to pH=7~8, add an appropriate amount of phase transfer catalyst, then raise the temperature to 110°C, drop in 1.05 mol potassium chloride based on phthalic anhydride, stir for 3 hours, and then wash with 5% NaCO3 solution. Separate the water layer and wash the oil layer with water; then the oil layer (crude ester) is distilled with water vapor to evaporate low-boiling substances such as alcohol, and then distilled under reduced pressure to collect the 220~222℃/660Pa fraction to obtain a white transparent oily liquid, i.e. Butylbenzyl phthalate.
Purpose
1. Mainly used as plasticizer. It has strong dissolving ability, good mutual solubility with polyvinyl chloride resin, vinyl acetate resin, polystyrene, and nitrocellulose. It has good pollution resistance, fast plasticizing speed, large filler capacity, and is water and oil resistant. Pull out. Can be used as the main plasticizer. It is often used in combination with other plasticizers for plastic floors, decorative materials, corrugated boards, etc. that contain a large amount of fillers. This product is used in the manufacture of films, sheets and pipes to obtain products with excellent transparency and smooth surfaces. It is also used in calendering French foam artificial leather.
2. Used as a plasticizer for water resistance, heat resistance, and oil extraction resistance. Good compatibility with most rubbers and resins, strong solvation, good heat resistance and light resistance, low volatility, resistance to pollution, fast plasticizing speed, low extraction of water and oil, large filling capacity and products It has the characteristics of good wear resistance, but poor plug resistance. It can be used as a plasticizer for polyvinyl chloride, vinyl chloride copolymer, cellulose resin, natural and synthetic rubber, and is used to manufacture highly filled plastic products such as floors, corrugated boards, sheets, and pipes. It can also be used with other plasticizers to make artificial leather and films to obtain excellent transparency and smooth surfaces. It is also suitable for post-foamed calendered products to avoid premature decomposition of the foaming agent.
