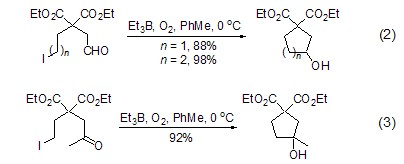
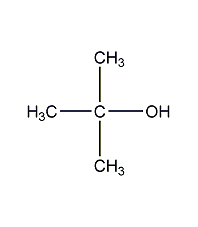
2,3-Butanedione-oxime
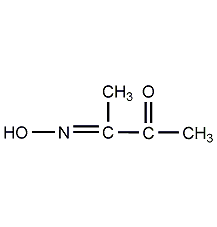
Structural formula
| Business number | 018Z |
|---|---|
| Molecular formula | C4H7NO2 |
| Molecular weight | 101.11 |
| label |
BDM, Biacetyl monoxime, Diacetyl monoxime, 2,3-Butanedione oxime, diacetyl oxime, Methyl oxime ethyl ketone, Dimethylethylenedione monooxime, Reagent |
Numbering system
CAS number:57-71-6
MDL number:MFCD00002116
EINECS number:200-348-5
RTECS number:EK3150000
BRN number:605582
PubChem number:24277826
Physical property data
1. Properties: White to light yellow crystalline powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 74℃ (76℃)
5. Boiling point (ºC, normal pressure): 185-186
6. Boiling point (ºC, normal pressure) 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation ( º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined Determined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17 . Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water , easily soluble in ethanol; ether and chloroform.
Toxicological data
1. Acute toxicity: mouse abdominal cavity LC50: 51mg/kg 2. Other multiple dose toxicity: rat abdominal cavity TDLo: 6850mg/kg/23D-I
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 25.36
2. Molar volume (cm3/mol): 93.9
3. Isotonic specific volume (90.2K): 224.8
4. Surface tension (dyne/cm): 32.7
5. Polarizability (10-24cm3): 10.05
CalculateAcademic data
1. Reference value for calculation of hydrophobic parameters (XlogP): 0.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 5
6. Topological molecular polar surface area (TPSA): 49.7
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 106
10. Isotopic atoms Quantity: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond positions Number of stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
with Ni2+ Pd2+ Co2+ ReO4– etc. form a light yellow or orange complex, which is easily extracted by chloroform.
Storage method
1. This product should be sealed and stored in a cool, dry place away from light.
2. The equipment in the production workshop must be sealed and the workshop must be well ventilated.
3. Packed in iron drums lined with plastic bags, placed in a cool and ventilated place to prevent heat and sunlight.
Synthesis method
1. Obtained from the reaction of methyl ethyl ketone and ethyl nitrite. Add methyl ethyl ketone and hydrochloric acid to the reaction pot, and immediately introduce ethyl nitrite gas. Keep the reaction at 40-55°C until the gas is exhausted. The ethanol generated by the reaction is evaporated under reduced pressure, and the distillation is stopped at 90°C to obtain diacetyl monooxime. The yield is 82-87%.
2.Mix methyl ethyl ketone and concentrated hydrochloric acid, heat to 40°C, introduce gaseous ethyl nitrite for reaction, and control the temperature at 40~ 45℃:
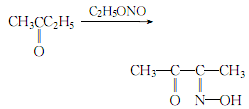
After the reaction is completed, heat and steam out the fraction below 90°C (ethanol generated by the reaction), and the residue is crude diacetyl monooxime. Neutralize the crude product with concentrated ammonia until the pH value is 6 to 7, then dilute it with 1/2 of its volume of water, and distill away the ethanol until the distillate cannot burn. ,
Switch to superheated steam distillation, add an appropriate amount of refined salt to the collected diacetyl monooxime distillate, cool to below 0°C to precipitate crystals, filter, spin dry, then recrystallize with water, and dry to obtain the pure product.
Purpose
1. Pharmaceutical intermediates. Analytical reagents.
2.Used for qualitative inspection Ni2+ and Ni2+, Co2+, Pt4+ and Pd2+ are also used for photometric determination of urea and ureide.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/low-odor-reactive-composite-catalyst-NT-CAT-9726-catalyst-9726.pdfextended-reading:https://www.bdmaee.net/niax-dmea-catalysts-dimethylethanolamine-momentive/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/31-10.jpgextended-reading:https://www.bdmaee.net/fomrez-ul-28-catalyst-dimethyltin-dioctadecanoate-momentive/extended-reading:https://www.cyclohexylamine.net/delay-catalyst-a-300-amine-catalyst-a-300/extended-reading:https://www.newtopchem.com/archives/44134extended-reading:https://www.newtopchem.com/archives/category/products/page/10extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyl-tin-maleate-CAS78-04-6-tributyl-tin-oxide.pdfextended-reading:https://www.newtopchem.com/archives/43932extended-reading:https://www.newtopchem.com/archives/category/products/page/118