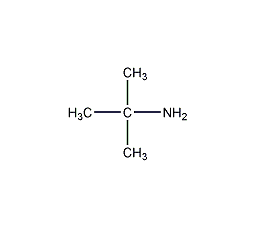
Structural formula
| Business number |
01JX |
| Molecular formula |
C4H11N |
| Molecular weight |
73 |
| label |
2-Aminoisobutane,
Trimethylaminomethane,
2-amino-2-methylpropane,
2-Amino-2-Methylpropane,
Trimethylaminomethane,
2-Aminisobutane
|
Numbering system
CAS number:75-64-9
MDL number:MFCD00008050
EINECS number:200-888-1
RTECS number:EO3330000
BRN number:605267
PubChem number:24864378
Physical property data
1. Properties: colorless transparent liquid with ammonia smell. [1]
2. Melting point (℃): -66.9[2]
3. Boiling point (℃): 44~46[3]
4. Relative density (water = 1): 0.696[4]
5. Relative vapor density (air=1): 2.5[5]
6. Saturated vapor pressure (kPa): 39.3 (20℃)[6]
7. Heat of combustion (kJ/mol): -2995.5 (liquid); -3025.2 (gas) [7]
8. Critical temperature (℃): 210.8[8]
9. Critical pressure (MPa): 3.84[9]
10. Octanol/water partition coefficient: 0.4[10]
11. Flash point (℃): -8.8[11]
12. Ignition temperature (℃): 380[12]
13. Explosion upper limit (%): 8.9[13]
14. Lower explosion limit (%): 1.7[14]
15. Solubility: soluble in water, soluble in absolute ethanol, benzene, chloroform, Most organic solvents such as ether. [15]
Toxicological data
1. Acute toxicity: human oral TCLO: 40 mg/m3/8H-I; rat LD50: 44mg/kg; rat inhalation LC50: 3800 mg/m3/4H; rat LD50: 450mg/kg; Mouse LD50: 378mg/kg; rabbit skin LD50: 2mg/kg; rabbit LD50: 375mg/kg;
2. Other multiple dose toxicity data: rat inhalation LC50: 2010mg/m3/6H/13W -I
3. Acute toxicity [16]
LD50: 44mg/kg (rat oral)
p>
LC50: 3800mg/m3 (rat inhalation, 4h)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 40h (theoretical).
4. Other harmful effects[18] This substance is harmful to the environment. Special attention should be paid to the pollution of water bodies. .
Molecular structure data
1. Molar refractive index: 24.08
2. Molar volume (cm3/mol): 98.2
3. Isotonic specific volume (90.2K ): 215.4
4. Surface tension (dyne/cm): 23.0
5. Polarizability (10-24cm3): 9.54
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 25.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties are similar to other primary amines. However, due to the steric effect of the tertiary carbon atom, the reaction is selective. For example, it reacts with ethylene oxide to form tert-butylaminoethanol, and then oxidizes it with potassium permanganate to obtain nitro-tert-butane. And the derivatives of tert-butylamine are more stable than the derivatives of butylamine and sec-butylamine. For example, it reacts with aldehydes to obtain stable Schiff base, and reacts with cyanogen chloride to obtain stable and distillable sec-butylaminocyanide.
2. Stability[19] Stable
3. Incompatible substances[20] Strong oxidants, strong acids, acids
4. Conditions to avoid contact[21] Heat
5. Polymerization hazard[22] No polymerization
6. Decomposition products[23] Ammonia
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Use tert-butyl alcohol and urea as raw materials to condense and hydrolyze in sulfuric acid to obtain tert-butyl urea, then neutralize with 40% sodium hydroxide, wash with water, filter dry, add liquid soda and ethylene glycol to mix After heating, collect the distillate at 40-60°C, then dry it with a reinforced alkali, fractionate under normal pressure, and cut the 44-47°C fraction to obtain the finished product of tert-butylamine. Raw material consumption quota: tert-butyl alcohol (98%) 2400kg/t, urea 2400kg/t, concentrated sulfuric acid (98%) 460kg/t. 2. Preparation of tert-butylamine by isobutylene-hydrocyanic acid method. First, isobutylene reacts with hydrocyanic acid to generate tert-butylamine sulfate, and then neutralizes with ammonia to obtain tert-butylamine, and at the same time, ammonium bisulfate is produced as a by-product. 3. According to relevant data reports, in addition to the above-mentioned preparation of tert-butylamine In addition to the law, there are several methods as follows: reduction method with bromine and potassium hydroxide using trimethylacetamide as raw material; co-heating method with tert-butyl chloride, ethanol and ammonia, etc.
2. Tert-butylamine is obtained by condensation and hydrolysis of tert-butyl alcohol.
(1) Condensation
The condensation of tert-butyl alcohol and urea gives tert-butyl urea.
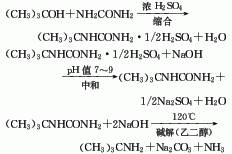
Add sulfuric acid to a dry reaction pot, stir and cool, and Slowly add urea at 15-25°C, maintain 20-25°C, then add tert-butyl alcohol, complete the addition, stir for 0.5h, and leave overnight. Put the reactants into ice water, add 20% sodium hydroxide solution to neutralize to pH=3~4, cool to 15~17°C, filter, wash with ice water, add water to the filter cake and boil to dissolve, filter while hot, cool the filtrate to Crystals precipitate at 0-5°C, filter and dry to obtain tert-butylurea.
(2) Hydrolysis

Sodium hydroxide solution, tert-butylurea, and ethylene glycol were added to the reaction pot, and heated to reflux for 4 hours. The reactant is distilled, and the 40-90°C fraction is collected to obtain crude tert-butylamine. Add a small amount of solid sodium hydroxide and dry overnight, perform crude distillation, and collect the 43-52°C fraction to obtain refined tert-butylamine.
The American Rohm-Haas Company uses isobutylene as raw material, reacts with hydrocyanic acid and neutralizes it with ammonia to obtain a product.
Purpose
1. Organic synthetic raw materials, used to synthesize medicines, rubber vulcanization accelerators, insecticides, fungicides and dye colorants, and can also be used as solvents. It can be used to produce rifampin, N-tert-butyl-2-benzothiazole sulfenamide, terbutylaminoethanol methacrylate, etc. N-tert-butyl-2-benzothiazole sulfenamide (accelerator NS) has a large production and consumption volume in the United States, accounting for 40% of the total volume of thiazole vulcanization chemicals (including vulcanizing agents and vulcanizing accelerators) %, accelerator NS is prepared by oxidative condensation of accelerator M or DM and tert-butylamine. Since the domestic production of tert-butylamine has not reached a large scale, the industrial production of the secondary additive NS has been affected.
2. Used as rubber accelerator, chemical reagent, and used to synthesize drugs, dyes, pesticides, etc. [25]
extended-reading:https://www.newtopchem.com/archives/44138extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/11.jpgextended-reading:https://www.cyclohexylamine.net/amine-catalyst-smp-delayed-catalyst-smp/extended-reading:https://www.newtopchem.com/archives/1899extended-reading:https://www.bdmaee.net/low-atomization-catalyst/extended-reading:https://www.cyclohexylamine.net/dabco-2033-dabco-tertiary-amine-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/78extended-reading:https://www.bdmaee.net/spraying-composite-amine-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/38.jpgextended-reading:https://www.bdmaee.net/dabco-tl-catalyst-cas10144-28-9-evonik-germany/