Tetramethylthiuram monosulfide
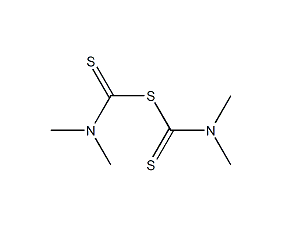
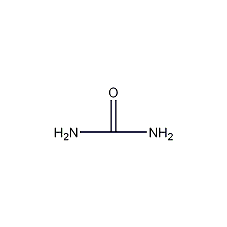
Structural formula
| Business number | 02CN |
|---|---|
| Molecular formula | C6H12N2S3 |
| Molecular weight | 208.37 |
| label |
Tetramethylthiodicarbonamide, Tetramethylthiuram monosulfide, Rubber accelerator TMTM, Accelerator TMTM, Bisdimethylsulfide, Bis(dimethylthioamide) sulfide, 4-methyl-thio-2 carbon 2 amide, 4-dimethyl sulfide thiuram, Rubber accelerator TMTM, PromoterTMTM, Pair of dimethyl sulfide, Sulfide Bis (2-methylthio amide), accelerator, Catalysts and auxiliaries |
Numbering system
CAS number:97-74-5
MDL number:MFCD00014870
EINECS number:202-605-7
RTECS number:WQ1750000
BRN number:1775650
PubChem number:24880353
Physical property data
1. Properties: yellow powder or granules
2. Relative density (g/mL, 25℃): 1.39-1.40
3. Relative vapor density (g/mL , air=1): Not determined
4. Melting point (ºC): 110
5. Boiling point (ºC, normal pressure): Not determined
6 . Boiling point (ºC, kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): 156
9. Ratio Optical rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, ºC): Not determined
p>
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble Soluble in water, gasoline, ethanol, benzene, acetone, chloroform.
Toxicological data
Acute toxicity: intraperitoneal – rat LD50: 383 mg/kg; oral – mouse LD50: 818 mg/kg
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 59.86
2. Molar volume (cm3/mol): 166.0
3. Isotonic specific volume (90.2K ): 465.9
4. Surface tension (dyne/cm): 62.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 23.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 96
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 147
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
2. Soluble in benzene, acetone, dichloroethane, carbon disulfide, toluene, chloroform, slightly soluble in ethanol and ether, insoluble in gasoline and water. Odorless, tasteless and non-toxic. Storage stable.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Add tetramethylthiuram disulfide (accelerator TMTD) and sodium cyanide to the reaction kettle, then add water and ethanol, and raise the temperature to 40-50°C with stirring to carry out desulfurization reaction. After the reaction is complete, cool down and let stand for stratification. After the oil layer is washed with water, separated and dehydrated, the finished product is obtained.
Purpose
Used as vulcanization accelerator for natural rubber and synthetic rubber.
extended-reading:https://www.cyclohexylamine.net/catalyst-pt303-high-efficiency-catalyst-pt303/extended-reading:https://www.bdmaee.net/niax-ef-712-low-emission-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/non-silicone-silicone-oil/extended-reading:https://www.newtopchem.com/archives/44045extended-reading:https://www.cyclohexylamine.net/high-quality-triethylenediamine-cas-280-57-9-dabco-teda/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/1-6.jpgextended-reading:https://www.bdmaee.net/polyurethane-delayed-catalyst-c-225-c-225-catalyst-c-225/extended-reading:https://www.cyclohexylamine.net/author/infobold-themes-com/extended-reading:https://www.newtopchem.com/archives/40394extended-reading:https://www.morpholine.org/high-quality-cas-108-01-0-nn-dimethyl-ethanolamine-2-dimethylamineethanol-dmea-dimethylethanolamine/