Chenodeoxycholic acid
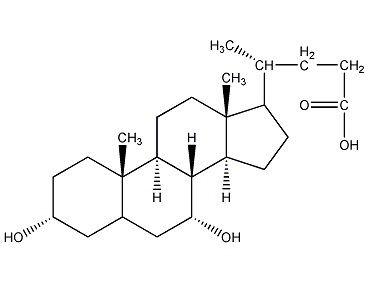

Structural formula
| Business number | 0514 |
|---|---|
| Molecular formula | C24H40O4 |
| Molecular weight | 392.57 |
| label |
Chenodeoxycholic acid, Chenodeoxycholic acid, 3alpha,7alpha-dihydroxy-5beta-cholanic acid, 3alpha,7alpha-Dihydroxy-5beta-cholanic acid, Chenodiol, CDCA, Lipoids |
Numbering system
CAS number:474-25-9
MDL number:MFCD00064142
EINECS number:207-481-8
RTECS number:FZ1980000
BRN number:3219887
PubChem number:24893153
Physical property data
1. Properties: White or light yellow crystalline powder, bitter taste 2. Density (g/m3, 25/4℃): Undetermined
3. Relative steam density (g/cm 3, air=1): Undetermined
4. Melting point (ºC): 141~1425. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation ( º): 12
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: in acetone, ethanol, Easily soluble in chloroform and glacial acetic acid, almost insoluble in water
Toxicological data
None yet
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 109.65
2. Molar volume (cm3/mol): 347.8
3. Isotonic specific volume (90.2K ): 905.9
4. Surface tension (dyne/cm): 46.0
5. Polarizability (10-24cm3): 43.46
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.9
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: None
6. Topological molecule polar surface area 77.8
7. Number of heavy atoms: 28
8 .Surface charge: 0
9. Complexity: 605
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 10
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters :0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Tightly sealed brown glass bottle. Store at low temperature.
Synthesis method
1. Take fresh or frozen chicken (or duck, goose) bile, add 1/10 of the industrial sodium hydroxide, heat and boil for 20 to 24 hours, and constantly replenish the evaporated water, cool, and then adjust the pH with hydrochloric acid When the value is 2 to 3, black paste appears. After standing for layering, take out the paste and wash it with water until it becomes neutral to obtain total bile acids. Add 2 times the amount of 95% ethanol and 10% activated carbon to the total bile acids, heat and reflux for 2 to 3 hours, and filter while hot. The filtrate is cooled, and then an equal volume of No. 120 gasoline is added for extraction and degreasing three times. The mixture is left to stand and separated into layers. The lower liquid is separated and concentrated under reduced pressure to obtain a paste. Add a large amount of water to the paste to precipitate, and wash the precipitate with water until it is colorless. Then add 2 times the amount of 95% ethanol and 5% sodium hydroxide solution to the precipitate, adjust the pH to 8.5, and heat to reflux for 2 hours. Then add barium chloride at an amount of 150g per liter, heat to reflux for 2 hours, filter while it is hot, and concentrate the filtrate until a crystal film or turbidity appears. Let it cool to separate the crystals, filter with suction, wash with water, and dry under reduced pressure to obtain white goose. Barium oxycholic acid salt crystals. Then dissolve the barium salt in water, add sodium carbonate containing about 12% of the barium salt, heat and stir, filter, discard the barium carbonate precipitate, adjust the filtrate to pH=2~3 with hydrochloric acid, precipitate, filter, and wash the filter cake with water until Neutral and dry, the finished product of chenodeoxycholic acid can be obtained. If necessary, recrystallize with ethyl acetate 1 to 2 times.
There is also a method of extracting calcium chloride salt.
Purpose
1. It can be used as a drug to dissolve gallstones, used to prevent and treat cholesterol gallstones and hyperlipidemia, and also has certain effects on pigmentary stones and mixed stones.
