Chloral Chloral
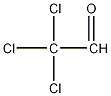
Structural formula
| Business number | 01KB |
|---|---|
| Molecular formula | C2HCl3O |
| Molecular weight | 147.38 |
| label |
Trichloroacetaldehyde, Trichloroacetaldehyde |
Numbering system
CAS number:75-87-6
MDL number:MFCD00036214
EINECS number:200-911-5
RTECS number:FM7870000
BRN number:506422
PubChem number:24853841
Physical property data
1. Properties: Colorless, easy-to-expand oily liquid with pungent odor. [1]
2. Melting point (℃): -57.5[2]
3. Boiling point (℃): 97.7[3]
4. Relative density (water = 1): 1.51[4]
5. Relative vapor Density (air=1): 5.1[5]
6. Saturated vapor pressure (kPa): 4.67 (20℃)[6]
7. Critical pressure (MPa): 4.45[7]
8. Octanol/water partition coefficient: 0.99[8]
9. Flash point (℃): 75[9]
10. Solubility: miscible in water, soluble in ethanol and ether , chloroform. [10]
Toxicological data
1. Acute toxicity Rat inhalation LC50: 440 mg/m3/4H; Mouse abdominal LD50: 600mg/kg; Dog inhalation LC50: 5900mg/m3/4H; Mammal caliber LD50: 710mg/kg;
2. Other multi-dose toxicity data Rat inhalation LC50: 80mg/m3/4H/2W-I;
3. Teratogenic Salmonella: 1mg/plate; Yeast: 1mg/L ;
4. Acute toxicity [11] LD50: 50~400mg/kg (rat oral)
5. Irritation[12] No data yet
6. Mutagenicity[13] Microbial mutagenicity: Salmonella typhimurium 1mg/dish
7. Carcinogenicity [14] IARC Carcinogenicity Comment: G3, for humans and insufficient evidence of carcinogenicity in animals.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[15] MITI-I test , initial concentration 100ppm, sludge concentration 30ppm, 8% degradation after 4 weeks.
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 26.01
2. Molar volume (cm3/mol): 93.0
3. Isotonic specific volume (90.2K ): 231.0
4. Surface tension (dyne/cm): 37.9
5. Polarizability (10-24cm3): 10.31
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 54.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14.The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances[17] Strong oxidizing agent, strong alkali
3. Conditions to avoid contact[18] Heating
4. Polymerization hazard[19] No polymerization
5. Decomposition products[20] Hydrogen chloride
Storage method
Storage Precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Produced by the reaction of ethanol or acetaldehyde and chlorine. Ethanol chlorination method: The stepwise reaction between ethanol and chlorine produces a mixture of alcoholic trichloroacetaldehyde, hydrated trichloroacetaldehyde and trichloroacetaldehyde, collectively called chlorine oil. React chlorine oil with concentrated sulfuric acid, and then use simple distillation to obtain refined trichloroacetaldehyde products. The by-product ethyl chloride can be packaged for the production of pesticides after washing with water, alkali washing, drying, condensation and distillation. Hydrogen chloride can be absorbed with water to produce 30% hydrochloric acid. Raw material consumption quota: ethanol (95%) 450kg/t, chlorine 2000kg/t, sulfuric acid 700kg/t.
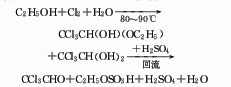
Purpose
1. Mainly used as raw materials for the manufacture of pesticides such as the insecticide DDT, trichlorfon, dichlorvos, and the herbicide trichloroacetaldehyde urea. In the pharmaceutical industry, it is used to produce chloramphenicol, mold compound, hypnotic agent trichloroacetaldehyde hydrate, etc. It can also be used as organic raw material to produce N,N-dimethylformamide, chloroform, chloroform, etc.
2. Used in DDT manufacturing and organic synthesis. [22]
