D-(-)-Penicillamine D-(-)-Penicillamine
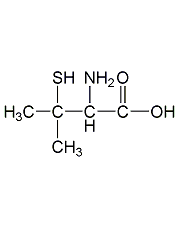

Structural formula
| Business number | 015L |
|---|---|
| Molecular formula | C5H11NO2S |
| Molecular weight | 149.21 |
| label |
D-Penicillamine, β-mercaptovaline, Penicillamine, dimethylcysteine, Penicamide, 3,3-Dimethyl-D(-)-cysteine, D-Penicillamine, 3,3-Dimethyl-D-cysteine, 3-Mercapto-D-valine, D-(−)-2-Amino-3-mercapto-3-methylbutanoic acid |
Numbering system
CAS number:52-67-5
MDL number:MFCD00064302
EINECS number:200-148-8
RTECS number:YV9425000
BRN number:1722375
PubChem number:24898564
Physical property data
1. Appearance: White crystalline powder,
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/ mL, air = 1): Undetermined
4. Melting point (ºC): 202~206
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): 169℃ (756mmHg)
7. Refractive index: 1.5310
8. Flash point (ºC): 73
9. Specific optical rotation (º,): 63° (C=0.1, in pyridine)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): 1.68mPa
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/ Log value of the distribution coefficient (water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, slightly soluble in ethanol, insoluble in chloroform and ether.
Toxicological data
The median lethal dose (rat, oral) is >10000mg/kg. The LD50 of intravenous injection in mice is 2289 mg, kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 38.13
2. Molar volume (cm3/mol): 123.8
3. Isotonic specific volume (90.2K ): 326.2
4. Surface tension (dyne/cm): 48.1
5. Polarizability (10-24cm3): 15.11
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 64.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 124
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Hydrochloride is easily hygroscopic, with a melting point of 177.5°C (decomposition). Allergy testing is required before use.
Storage method
Should be sealed and stored dry at 4°C.
Synthesis method
Produced by degradation or synthesis of penicillin. It is derived from the hydrolysis of penicillin G potassium salt.
Purpose
It is a detoxifying drug for copper, mercury, copper and other heavy metals. It is also used to treat autoimmune diseases such as hepatolenticular degeneration (Wilson’s disease) and rheumatoid arthritis caused by copper deposition in tissues. It is contraindicated in patients with penicillin allergy, hematopoietic dysfunction, and renal insufficiency.
