Dicyclohexyl ketone
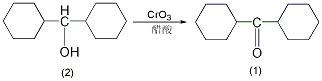
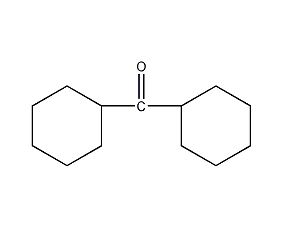
Structural formula
| Business number | 03B3 |
|---|---|
| Molecular formula | C13H22O |
| Molecular weight | 194.31 |
| label |
(C6H11)2CO, Alicyclic compounds |
Numbering system
CAS number:119-60-8
MDL number:MFCD00040417
EINECS number:204-336-0
RTECS number:OB1804500
BRN number:None
PubChem number:24894184
Physical property data
None yet
Toxicological data
1. Acute toxicity: mouse intravenous LD50: 56mg/kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 57.91
2. Molar volume (cm3/mol): 200.2
3. Isotonic specific volume (90.2K): 492.8
4. Surface tension (dyne/cm): 36.7
5. Polarizability (10-24cm3): 22.95
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 165
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
1. Preparation method:
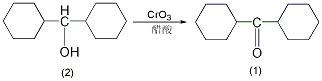
Add 10g (0.051mol) of dicyclohexylmethanol (2) and 50mL of glacial acetic acid into the reaction bottle. Slowly add 11g of chromic anhydride (0.11mol) while stirring. Maintain the reaction temperature at 20~30°C and add dicyclohexylcarboxylic acid dropwise. After adding a mixture of 22g methanol (0.112mol) and 80mL glacial acetic acid, stir and react at room temperature for 0.5h. Slowly raise the temperature to 75°C and react at 75°C for 0.5h. Cool and adjust the pH to above 10 with 20% sodium hydroxide aqueous solution. Extract with diethyl ether (50mL×3), combine the diethyl ether extracts, wash with water, and dry over anhydrous sodium sulfate. Filter, evaporate the ether, and then distill under reduced pressure to collect the fraction at 155~158℃/2.1kPa to obtain 26g of dicyclohexylmethylketone (1), with a yield of 81%. nD171.4850.[1]
Purpose
Temporarily�
