Diethyl disulfide Diethyl disulfide
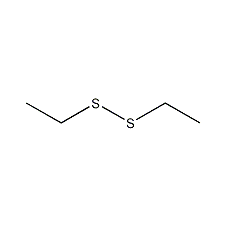

Structural formula
| Business number | 0325 |
|---|---|
| Molecular formula | C4H10S2 |
| Molecular weight | 122 |
| label |
Ethyl disulfide, diethyl disulfide, diethyl disulfide, Ethylene disulfide, diethyl disulfide, 1-(Ethyldisulfanyl)ethane, Diethyl disulphide, 3,4-Dithiahexane |
Numbering system
CAS number:110-81-6
MDL number:MFCD00009266
EINECS number:205-710-6
RTECS number:JO1925000
BRN number:1098273
PubChem number:24894475
Physical property data
1. Properties: colorless or light yellow oily substance with foul odor.
2. Density (g/mL, 20℃): 0.993
3. Relative vapor density (g/mL, air=1): 5.9
4. Melting point (ºC): -102
5. Boiling point (ºC, normal pressure): 152-154
6. Boiling point (ºC, 5.2kPa): Undetermined
p>
7. Refractive index (n20D): 1.506
8. Flash point (ºC ): 40
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11 . Vapor pressure (mmHg, 25ºC): Undetermined
12. Saturation vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/water) Log value of distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, easily soluble in organic solvents such as ethanol and ether.
Toxicological data
1. Irritation: Rabbit eye: 100mg/24H, moderate irritation.
2. Acute toxicity: Rat oral LD50: 2030 mg/kg
Acute toxicity:
Oral LD50: 2030mg/kg(rat)
Irritation to eyes moderate 100mg/24H(rbt)
Main irritating effects:
On skin: May cause inflammation.
On the eyes: Irritation effects.
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard class 1 (German Regulation) (self-assessment via list) This substance is slightly hazardous to water.�
Do not allow dilute or large quantities of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 36.57
2. Molar volume (cm3/mol): 122.5
3. Isotonic specific volume (90.2K): 292.6
4. Surface tension (dyne/cm): 32.5
5. Polarizability (10-24cm3): 14.49
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 50.6
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 17.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It does not decompose under normal temperature and pressure, and avoid contact with oxidants.
2. Exist in mainstream smoke.
Storage method
Store in a cool, dry and ventilated warehouse. Keep away from fire, heat sources and anti-static. The storage temperature should not exceed 30℃. The packaging must be sealed and must not come into contact with oxidants. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The preparation method is to first put sodium sulfide solution into the reaction kettle, add sulfur powder according to sodium sulfide: sulfur = 1:1 (molar ratio), heat up the jacket, and keep it warm under stirring when the temperature reaches 80 to 95°C. 1 hour to prepare disodium disulfide (Na2S2) solution, then pump the disodium disulfide solution into the alkylation kettle, start stirring, and wait until the reaction kettle becomes vacuum When the temperature reaches (2~3.3)×104Pa, close the vacuum valve and start to flow in ethyl chloride. The reaction temperature is controlled at 75~90℃ and the pressure is controlled at (1.17~1.18)×105 Pa, the feeding ratio (molar ratio) is disodium disulfide:ethyl chloride=1:2. After passing the ethyl chloride, cool the material to below 50°C, and pump the oil to the static water separator. Set the layers, and the upper oil layer is the finished product.
2C2H5Cl+Na2S+S→C2H 2—S—S—C2H5+2NaCl
Purpose
Used as intermediates in organic synthesis.
