Diethyl ethoxymethylenemalonate
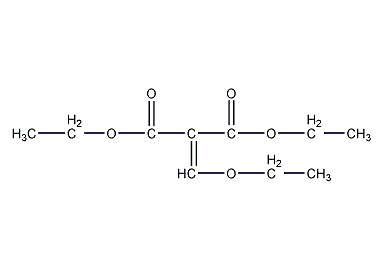
Structural formula
| Business number | 01XK |
|---|---|
| Molecular formula | C10H16O5 |
| Molecular weight | 216.23 |
| label |
Diethyl Ethoxymethylenemalonate, Diethyl ethoxybutenone, Diethyl (ethoxymethylene)malonate, C2H5OCH=C(COOC2H5)2 |
Numbering system
CAS number:87-13-8
MDL number:MFCD00009148
EINECS number:201-725-7
RTECS number:OO1100000
BRN number:880058
PubChem number:24845377
Physical property data
1. Properties: liquid
2. Relative density (25/4℃): 1.07
3. Boiling point (℃): 279~281
4. Refractive index (20℃): 1.4620
5. Flash point (℃): 155
Toxicological data
1. Acute toxicity
Rat caliber LD50: 925mg/kg;
Mouse caliber LD50: 2227mg/kg; Mouse inhalation LC50: 44mg/m3
2. Neurotoxicity
Rabbit skin test: 500 mg/24HREACTION
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 53.57
2. Molar volume (cm3/mol): 200.3
3. Isotonic specific volume (90.2K ): 482.5
4. Surface tension (dyne/cm): 33.6
5. Polarizability (10-24cm3):21.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 61.8
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 224
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Skin contact may cause allergic reactions. After contact with skin, wash with plenty of soap immediately. Operators should wear protective equipment.
Storage method
This product should be kept sealed, dry and protected from light.
Synthesis method
Stir and heat the mixture of triethyl orthoformate, acetic anhydride, diethyl malonate and anhydrous zinc oxide, keep it at 102-115℃ for 2.5h, and at 115-127℃ for 7h, then add some Acetic anhydride and triethyl orthoformate were heated to 127-145°C for 2 hours, and then kept at 145-155°C for 2 hours. The reaction was then cooled to room temperature and filtered. The filtrate is distilled under reduced pressure, and the 108-110°C (33Pa) fraction is collected to obtain the finished product with a yield of 50-60%.
![]()
Purpose
This product is an important organic raw material containing a variety of active groups in the molecule. It can perform polycondensation and cyclization condensation with a variety of substances. It is now mainly used to synthesize norfloxacin.
