Diethyl ethyl phosphate Diethyl ethylphosphonate

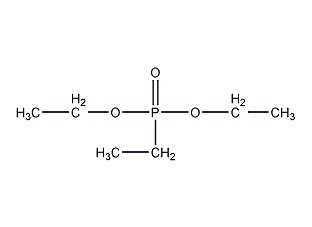
Structural formula
| Business number | 01MZ |
|---|---|
| Molecular formula | C6H15O3P |
| Molecular weight | 166.16 |
| label |
Diethylpropionic aldehyde, Diethoxyethylphosphine oxide, ethyl-phosphonic acidiethylester, Highly efficient organophosphorus flame retardant |
Numbering system
CAS number:78-38-6
MDL number:MFCD00039888
EINECS number:201-111-9
RTECS number:SZ7925000
BRN number:1756861
PubChem number:24869763
Physical property data
1. Physical property data
1. Properties: colorless and transparent liquid.
2. Density (g/mL, 25/4℃): 1.028
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): Uncertain
5. Boiling point (ºC, normal pressure): 200
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: 1.412~1.418 (25℃)
8. Flash point (ºC): >200
9. Specific rotation (º) : Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion Upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Compatible with water and organic solvents Miscible. .
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 40.21
2. Molar volume (cm3/mol): 164.4
3. Isotonic specific volume (90.2K): 382.3
4. Surface tension (dyne/cm): 29.2
5. Polarizability (10-24cm3): 15.94
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 35.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 114
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Add absolute ethanol, methyl red and solvent to the reaction kettle, and evenly add the phosphorus trichloride and solvent mixture to the high tank. Pour ammonia gas into the kettle at about 0°C while stirring, and add phosphorus trichloride dropwise at the same time. Adjust the feeding speed of the two to keep the reaction temperature at 0 to 10°C. Complete the dropwise addition of phosphorus trichloride in about 2 to 3 hours. Continue to pass ammonia for a few minutes to make the reactant alkaline (pH 8 to 9). Add water to dissolve the by-product ammonium chloride and a small amount of diethyl phosphite, and let it stand to separate the water layer. The oil layer is fractionated under reduced pressure to recover the solvent. Then use vacuum fractionation to collect triethyl phosphite. The boiling point is 52~53°C (1999.8Pa). , the yield is 70% to 75%. The reaction solvent is preferably petroleum ether at about 90°C. Trichlorethylene, toluene, and xylene can also be used. Triethyl phosphite undergoes an isomerization reaction under the action of a catalyst to produce diethyl ethylphosphonate.

Purpose
This product is a new type of highly efficient organophosphorus flame retardant. It is widely added to various stearic foam plastics and has better flame retardant effect. This product has low viscosity, does not contain halogen, and is very stable in a two-component system of polyether polyol and isocyanate.
