Diethyl succinate Diethyl succinate
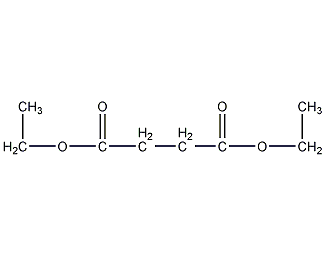

Structural formula
| Business number | 03GC |
|---|---|
| Molecular formula | C8H14O4 |
| Molecular weight | 174.19 |
| label |
diethyl succinate, Ethylsuccinate, Diethyl butanedionate, gas chromatography stationary solution, food additives, aliphatic compounds |
Numbering system
CAS number:123-25-1
MDL number:MFCD00009208
EINECS number:204-612-0
RTECS number:WM7400000
BRN number:907645
PubChem number:24846069
Physical property data
1. Properties: Colorless liquid with a faint pleasant aroma.
2. Boiling point (ºC, 101.3kPa): 217.7
3. Boiling point (ºC, 1.463kPa): 105
3. Melting point (ºC): -21
4. Relative density (g/mL, 20/4ºC): 1.0402
5. Refractive index (20ºC): 1.4201
6. Flash Point (ºC): 90
7. Solubility: Miscible with ethanol and ether, soluble in acetone, insoluble in water.
8. Relative density (25℃, 4℃): 1.0349
9. Refractive index at room temperature (n25): 1.415430
10. Critical temperature (ºC): 389.85
11. Liquid phase standard hot melt (J·mol-1·K-1): 332.3
Toxicological data
1. Acute toxicity: Rat oral LD50: 8530mg/kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 42.66
2. Molar volume (cm3/mol): 167.5
3. Isotonic specific volume (90.2K ): 399.8
4. Surface tension (dyne/cm): 32.4
5. Polarizability (10-24cm3): 16.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 135
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and Stability
1. It is flammable and will burn easily when exposed to open flames or high heat.
2. Found in tobacco leaves.
Storage method
None yet
Synthesis method
1. It is prepared by acylation of ethanol and succinic acid; or it is prepared by direct esterification of ethanol and succinic acid in benzene solution in the presence of concentrated sulfuric acid.
2. Preparation method:
In a reaction bottle equipped with a distillation device, add 58g (0.5mol) of succinic acid (2) and 82g of absolute ethanol (1.76mol) , 180mL of benzene dried with metallic sodium, one grain of zeolite, 11mL of sulfuric acid. Heat and reflux for 8 hours. The reactants were poured into excess water, the organic layer was separated, and the aqueous layer was extracted twice with benzene. The organic layers were combined, washed with a small amount of water, and then washed with saturated sodium carbonate solution. Dry over anhydrous sodium sulfate. Fractionate under reduced pressure and collect the fractions at 80~83℃/400Pa to obtain 75g of diethyl succinate (1) with a yield of 85%. [1]
Purpose
1. Used as solvent, gas chromatography stationary solution, and food additive.
2. It plays the role of fixative and solution in essence.
