Diisoamyl ether

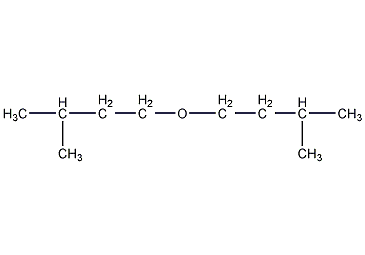
Structural formula
| Business number | 05M9 |
|---|---|
| Molecular formula | C10H22O |
| Molecular weight | 158.29 |
| label |
1,1′-Oxobis(3-methylbutane), Isoamyl ether, Diisopentyl Ether, Isoamyl Ether, Isopentyl Ether, Ether solvents, Extracting agent, Odor gas absorbent, rubber regeneration solvent, alkaloid solvent |
Numbering system
CAS number:544-01-4
MDL number:MFCD00008947
EINECS number:208-857-4
RTECS number:EK5433750
BRN number:1698014
PubChem number:24890223
Physical property data
1. Properties: colorless liquid with slight fruity aroma. [1]
2. Melting point (℃): -69.3[2]
3. Boiling point (℃): 172.5[3]
4. Relative density (water = 1): 0.778[4]
5. Relative vapor Density (air=1): 5.46[5]
6. Saturated vapor pressure (kPa): 0.19 (25℃)[6]
7. Octanol/water partition coefficient: 4.25[7]
8. Flash point (℃): 45.56[8]
9. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, ether, chloroform, acetone and so on. [9]
10. Refractive index (n20ºC): 1.4085
11. Viscosity (mPa·s, 11ºC): 1.401
12. Heat of evaporation (KJ/mol): 35.17
13. Specific heat capacity (KJ/(kg·K)): 2.40
14. Vapor pressure (kPa, 18.6ºC ): 0.13
15. Vapor pressure (kPa, 57.0ºC): 1.33
16. Vapor pressure (kPa, 109.6ºC): 13.33
17. Vapor pressure (kPa, 129.0ºC): 26.67
18. Vapor pressure (kPa, 150.3ºC): 53.33
Toxicological data
1. Acute toxicity[10] LD50: >10g/kg (oral in mice)
2. Irritation No information available
Ecological data
1. Ecotoxicity[11] LC50: 4.7mg/L (48h) (medaka)
2. Biodegradability No information available
3. Non-biodegradability[12] In the air, when the hydroxyl radical concentration is 5.00×105/cm3, the degradation half-life is 14h (theoretical).
4. Bioconcentration[13] BCF: 117~313 (carp, contact concentration 60ppb, contact Time 8 weeks); 84~260 (carp, exposure concentration 6ppb, exposure time 8 weeks)
Molecular structure data
1. Molar refractive index: 50.04
2. Molar volume (cm3/mol): 200.7
3. Isotonic specific volume (90.2K): 444.4
4. Surface tension (dyne/cm): 24.0
5. Polarizability (10 -24cm3): 19.83
6. Dielectric constant (20ºC): 2.82
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 66.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the general chemical properties of aliphatic ethers. It is stable to acids and alkalis, but can undergo auto-oxidation when in contact with air to generate peroxide.
2. Stability[14] Stable
3. Incompatible substances[15] Strong oxidants, strong acids, halogens, sulfur, sulfides
4. Conditions to avoid contact [16] Air , light
5. Aggregation hazard[17] No aggregation
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. The storage temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:

Refer to the above preparation method of n-amyl ether (reference book page 242), use 58.7g of 3-methyl-1-butanol (2) (72mL, 0.67mol), 4.5mL of concentrated sulfuric acid, a few grains of zeolite, and the temperature rises to 148~150°C, and collect the fractions at 135~150°C (16g), 150~168°C (12g) and 168~174°C (12g). After treatment with metallic sodium and re-distillation, 28g of diisoamyl ether (1) was obtained with a yield of 53%. [20]
Purpose
1. Perfume raw materials, Grignard reaction solvents, solvents used to prepare metal catalysts for organic synthesis by extraction methods, odor gas absorbers, rubber regeneration solvents, and alkaloid solvents. Used as an extraction agent for oils and alkaloids and a solvent for Grignard reaction.
2. Used as solvent and in paint and recycled rubber industries. [19]
