Diphenyl sulfone Diphenyl sulfone
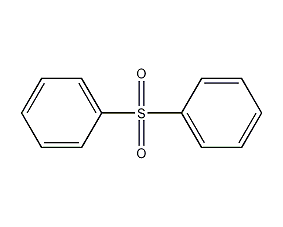

Structural formula
| Business number | 03L1 |
|---|---|
| Molecular formula | C12H10O2S |
| Molecular weight | 218.27 |
| label |
diphenyl sulfone, 1,1′-Sulfonyldiphenyl, 1,1-Sulfonylbiphenyl, diphenyl sulfone, phenylsulfone, Phenyl sulfone, acaricides, Plasticizer |
Numbering system
CAS number:127-63-9
MDL number:MFCD00007548
EINECS number:204-853-1
RTECS number:SX2400000
BRN number:1910573
PubChem number:24868937
Physical property data
1. Properties: white crystal powder
2. Relative density (20/4℃): 1.252
3. Melting point (℃): 127~130
4. Boiling point (ºC, 101.325kpa): 379
5. Solubility: Dissolved in organic solvents.
Toxicological data
1. Acute toxicity: Rice LD5O: 2250mg/Kg
Rat inhale LC:> 1700mg/M 3 /8h
Mouse oral LD5O: 375mg/kg
Mouse peritoneal cavity LD5O: 313mg/kg
Mouse subcutaneous LD5O: 329mg/kg
Mouse Intravenous injection of LD5O: 320mg/kg
Oral LDLO in rabbits: 4700mg/kg
2. Other multi-dose toxicity: Oral TDLO in rats: 4500mg/kg/2W-I
Ecological data
None
Molecular structure data
1. Molar refractive index: 60.27
2. Molar volume (cm3/mol): 177.7
3. Isotonic specific volume (90.2K ): 457.1
4. Surface tension (dyne/cm): 43.6
5. Polarizability (10-24cm3): 23.89
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 42.5
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 253
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Prevent contact with eyes, skin and clothing, and prevent accidental ingestion and inhalation.
Storage method
Store in a cool, dry place. Keep lid tightly closed when not in use.
Synthesis method
Benzene sulfonic acid is produced by the sulfonation of benzene, and then reacted with benzene. First, heat 100% sulfuric acid to 130ºC, and introduce benzene vapor into it. After 20 minutes, the reaction temperature rises to 180ºC, and then after 2 hours, The reaction temperature is increased to 205~215ºC and the reaction is continued for 10~12h. After the reaction stops, cool, pour into water, filter, wash and dry to obtain the finished product.
Purpose
Used in organic synthesis as plasticizer and acaricide.
Used as pharmaceutical and pesticide intermediates.
