DL-glyceraldehyde DL-Glyceraldehyde
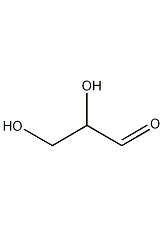
Structural formula
| Business number | 017X |
|---|---|
| Molecular formula | C3H6O3 |
| Molecular weight | 90.08 |
| label |
None yet |
Numbering system
CAS number:56-82-6
MDL number:MFCD00064379
EINECS number:200-290-0
RTECS number:MA6475000
BRN number:635685
PubChem number:24895192
Physical property data
1. Properties: white crystal.
2. Density (g/mL, 25/4℃): 1.445
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 145
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, insoluble in benzene and petroleum ether and pentane.
Toxicological data
1. Acute toxicity: rat abdominal LD50: 2mg/kg 2. Mutagenicity: mutation microorganismsTEST system: bacteria – Salmonella typhimurium: 100ug/plate
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 19.16
2. Molar volume (cm3/mol): 70.7
3. Isotonic specific volume (90.2K): 191.2
4. Surface tension (dyne/cm): 53.3
5. Polarizability (10-24cm3): 7.59
Calculate chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): -1.6
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 43.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine ��Number of stereocenters of chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be stored in a sealed, cool place and away from light.
Synthesis method
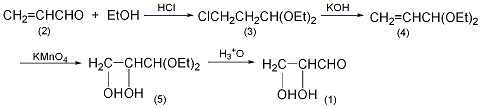
1. Mix 50g (0.3mol) diethanol acetal DL-glyceraldehyde and 500ml 0.1mol/L sulfuric acid, and place at 20°C for 7 days. After adding 30ml of glacial acetic acid, neutralize with barium hydroxide solution, add 5g of activated carbon, stir and filter. The water in the filtrate was evaporated under reduced pressure, and an equal volume of absolute ethanol was added to the residue. The crystals were filtered out and dried to obtain 22g DL-glyceraldehyde, with a yield of 80%.
2. Preparation method:

Dietol-β-chloroacetaldehyde (3): In a 3L reaction bottle equipped with a stirrer and ventilation tube, add 253 mL of absolute ethanol, cool it to below 0°C in an ice-salt bath, and dry it of hydrogen chloride gas to saturation. While stirring, 112g (2.0 mol) of acrolein (2) cooled to 0°C was added dropwise, the reaction temperature was controlled to 0°C, and the addition was completed in about 1.5 hours. Let stand and separate the layers, separate the lower organic layer, add solid sodium bicarbonate powder in batches, neutralize and remove acid. Filter, wash the filtrate with ice water, and dry over anhydrous potassium carbonate for 10 hours. After filtration, distill under reduced pressure and collect the fractions at 58-62°C/1.06kPa to obtain 112g of diethanol-β-chloroacetaldehyde (3) with a yield of 34%. Diethanol acrolein (4): Add 340g (6mol) of dry powdered potassium hydroxide and 167g (1.0mol) of the above compound (3) into a distillation bottle. After shaking vigorously, install a fractionation device. Heat the distillation bottle in an oil bath at 210 to 220°C until no more distillate is distilled. The water in the distillate was separated, and the organic layer was dried with potassium carbonate, filtered and distilled. The fractions at 122-126°C were collected to obtain 98g of diethanol acrolein (4), with a yield of 75%. Diethylglyceraldehyde (5): In a reaction bottle equipped with a stirrer, thermometer, and dropping funnel, add 65g (0.5mol) of diethylglyceraldehyde (4) and 600mL of water, cool to 5°C, and stir. Add dropwise a solution of 80g (0.5mol) water, control the dropping speed to about 25mL/min, and keep the temperature of the reaction solution at about 5°C. Stirring – stop, the reactants will become gel-like. After leaving it for 2 hours, heat it in a steam bath for 1 hour. Filter with suction, and wash the manganese dioxide with 150 mL of water. Combine the filtrate and washing liquid, and add 1200g anhydrous potassium carbonate while cooling. The organic layer was separated, and the aqueous layer was extracted 4 times with diethyl ether. The organic layers were combined and dried over anhydrous potassium carbonate. After recovering the diethyl ether, distill under reduced pressure and collect the fraction at 120-121°C/1.06kPa to obtain 55g of diethyl glyceraldehyde (5) with a yield of 67%. DL-Glyceraldehyde (1): Place 50g (0.3mol) of the above-mentioned diethanol glyceraldehyde (5) and 0.05mol/L sulfuric acid in a reaction bottle, and place it at 20°C for 1 week. Add 30 mL of glacial acetic acid. The reaction compound was carefully neutralized with barium hydroxide solution and decolorized with activated carbon. Filter, and the filtrate is concentrated at a pressure of 1.33kPa. Add an equal volume of absolute ethanol to the remainder and slowly crystallize. Filter the precipitated crystals and vacuum dry them in a vacuum dryer containing soda lime and calcium chloride desiccant to obtain 22g of DL-glyceraldehyde (1) with a yield of 80%. [1]
Purpose
Used in biochemical research, organic synthesis intermediates, and nutritional agents.
