Durene Durene
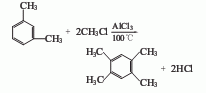
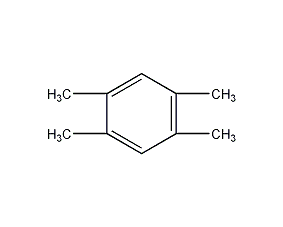
Structural formula
| Business number | 02A6 |
|---|---|
| Molecular formula | C10H14 |
| Molecular weight | 134.22 |
| label |
1,2,4,5-Tetramethylbenzene, Durene, 1,2,4,5-Tetramethylbenzene, Durol, hydrocarbon solvents, aromatic compounds, For synthesis of pyromellitic acid |
Numbering system
CAS number:95-93-2
MDL number:MFCD00008528
EINECS number:202-465-7
RTECS number:DC0500000
BRN number:1903393
PubChem number:24900002
Physical property data
1. Properties: White or colorless crystals with a camphor-like smell.
2. Density (g/mL, 81/4℃): 0.84
3. Relative vapor density (g/mL, air=1): 4.6
4. Melting point (ºC): 79.24
5. Boiling point (ºC, normal pressure): 196.8
6. Flash point (ºC): 73
7. Vapor pressure (mmHg, 140ºC): 160
8. Saturated vapor pressure (kPa, 128.1ºC): 13.33
9. Critical temperature (ºC): 402.5
10. Solubility: Insoluble in water, soluble in ethanol, ether, benzene and acetone.
11. Critical pressure (MPa): 2.9
12. Eccentricity factor: 0.435
13. Crystal phase standard entropy (J·mol– 1·K-1): 416.6
14. Crystal phase standard hot melt (J·mol-1·K-1): 183.1
15. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5837.27
16. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -98.66
17. Liquid phase standard entropy (J·mol-1 ·K-1): 300.54
18. Liquid phase standard free energy of formation (kJ·mol-1): 101.35
19. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5893.50
20. Gas phase standard claimed heat (enthalpy) (kJ ·mol-1): -42.43
21. Gas phase standard entropy (J·mol-1·K-1): 422.8
22. Gas phase standard free energy of formation (kJ·mol-1): 121.14
23. Refractive index (n 81D): 1.479
Toxicological data
Acute toxicity: Rat oral LD50: 6989mg/kg; mouse intravenous LD50: 180mg/kg
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment. Can cause pollution to water bodies and the atmosphere.
Molecular structure data
1. Molar refractive index: 45.55
2. Molar volume (cm3/mol): 154.5
3. Isotonic volume (90.2K): 357.8
4. Surface tension (dyne/cm): 28.7
5. Dielectric constant: 2.41
6 , Dipole moment (10-24cm3):
7. Polarizability: 18.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 80.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants. Flammable. Avoid inhaling the dust of this product and avoid contact with eyes and skin.
2.This product is moderately toxic. LD50 after oral administration: 3.4g/kg for mice and 6.7g/kg for rats. It can make animals lethargic and inhibit the excitability of the central nervous system. It has minimal irritation to the skin, does not cause allergies, and has no signs of absorption through the skin. The maximum allowable concentration in the air is 50 mg/m3.
3. Exist in smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
2. Pack in iron drums lined with plastic bags, store in a ventilated, dry place, moisture-proof, sun-proof, and away from fire sources. Store and transport according to regulations on toxic chemicals.
Synthesis method
In industry, tetramethylbenzene has been produced from C9-C10 aromatic hydrocarbon fractions by distillation to remove naphthalene, then frozen (-70°C) and recrystallized. But the cost is higher. Nowadays, it is produced by synthetic method. For example, m-xylene is used as raw material, anhydrous aluminum trichloride is added, methyl chloride is introduced at 100°C for reaction, then left to stand and cooled, and the upper dark green oily substance is separated. After drying, Distill under normal pressure and collect the 180-205°C fraction. In addition, there are also methods for producing tetramethylbenzene by using isomerization, alkylation, conversion alkylation, disproportionation-isomerization of xylene and trimylene.
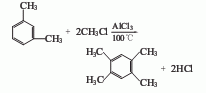
Purpose
Used in organic synthesis, plasticizer, and preparation of pyromellitic dianhydride. Used as reagent and raw material of pyromellitic acid.
