Ethyl isovalerate Ethyl isovalerate
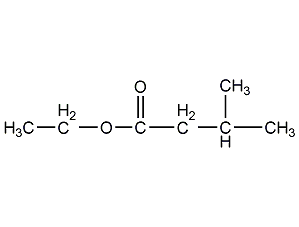

Structural formula
| Business number | 02XM |
|---|---|
| Molecular formula | C7H14O2 |
| Molecular weight | 130.18 |
| label |
3-Methylbutyrate ethyl ester, Isovaleric acid ethyl ester, Ethyl 3-methylbutyrate, Spices raw materials |
Numbering system
CAS number:108-64-5
MDL number:MFCD00009203
EINECS number:203-602-3
RTECS number:NY1504000
BRN number:1744677
PubChem number:24886231
Physical property data
1. Properties: colorless oily liquid with fruity aroma. [1]
2. Melting point (℃): -99.3[2]
3. Boiling point (℃): 132~135[4]
4. Relative density (water=1): 0.864[5]
5. Saturated vapor pressure (kPa): 0.56 (20℃)[6]
6. Heat of combustion (kJ/mol): -4180[7]
7. Critical temperature (℃): 314.8[8]
8. Critical pressure (MPa): 2.84[9]
9. Octanol/water partition coefficient: 2.26[10]
10. Flash point (℃): 25[ 11]
11. Solubility: slightly soluble in water, miscible in ethanol, ether, benzene, and soluble in propylene glycol. [12]
12. Refractive index (n20ºC): 1.3962
13. Refractive index (n25ºC): 1.3944
14 . Heat of evaporation (KJ/mol, 25ºC): 47.3
15. Heat of evaporation (KJ/mol, b.p.): 37.0
16. Heat of formation (KJ/mol, 25ºC) :-540.1
17. Solubility parameter (J·cm-3)0.5: 16.572
18. van der Waals area (cm2·mol-1): 1.183×1010
19. van der Waals volume ( cm3·mol-1): 83.450
20. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1): -4184.4
21. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -570.9
22. Liquid phase standard hot melt (J·mol-1·K-1): 256.2
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 500mg/24H, severity of reaction: mild.
2. Acute toxicity: rat oral LD50: 1200mg/kg; mouse oral LD50: 7031mg/kg;
3. Inhalation, oral administration or skin absorption to the body harmful.
4. Acute toxicity [13]
LD50: 1200mg/kg (rat abdominal cavity); 7031mg/kg ( Rabbit oral)
5. Irritation [14] Rabbit transdermal: 500mg (24h), mild irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[15] This substance may be harmful to the environment and is harmful to the environment. Bodies of water should be given special attention.
Molecular structure data
1���Molar refractive index: 36.21
2. Molar volume (cm3/mol): 147.9
3. Isotonic specific volume (90.2K): 332.7
4. Surface tension (dyne/cm): 25.5
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 14.35
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 86.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the general chemical properties of ester and is easily hydrolyzed in the presence of caustic alkali.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, strong acids, strong bases
4. Polymerization hazards[18] No polymerization
Storage method
Storage Precautions[19] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Direct esterification of isovaleric acid and ethanol under the catalysis of sulfuric acid. After the reaction mixture is cooled, it is neutralized with sodium carbonate, washed with water, dried and fractionated to obtain the product.
(CH3)2CHCH2COOH+CH3CH2OH[H2SO4]→(CH3)2CHCH2COOCH2CH3+H2O
Isobutyronitrile and ethanol can also be heated and refluxed for 10 hours under the catalysis of sulfuric acid, and then neutralized and washed with water to separate the ester layer. Finally, the finished product is obtained by fractionation.
Refining method: washing with saturated sodium carbonate solution, drying with anhydrous sodium sulfate or magnesium sulfate and then distilling.
2. It is obtained by direct esterification of isovaleric acid and ethanol under the catalysis of sulfuric acid, followed by washing, drying and fractionation. It can also be obtained by heating reflux of isobutyronitrile and ethanol under the catalysis of sulfuric acid, followed by neutralization, water washing, separation and fractionation.
Purpose
1. This product has an aroma similar to apples and mulberry, and can be used as essential oils, spices, artificial fruit flavors, and can also be used in food and tobacco. This product can also be used as a solvent.
2. Used to prepare apples, strawberries, pineapples, bananas, nuts, grapes, pears, tropical fruits and other food flavors and tobacco flavors; it is also used in small amounts in daily chemical flavors to act as a pre-flavor. . The usage amount in gum is 80~430mg/kg; in candies 29mg/kg; in baked goods 27mg/kg; in cold drinks 7.5mg/kg; in puddings 5.0mg/kg; in soft drinks 4.9mg/kg.
3. Used as flavor. [20]
