Ethyl propionate Ethyl propionate
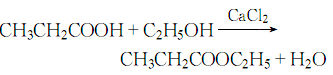

Structural formula
| Business number | 02RK |
|---|---|
| Molecular formula | C5H10O2 |
| Molecular weight | 102.13 |
| label |
Ethyl oleate, Butyl phthaloyl glycolate, Ethyl n-propionate, Propionic acid ethyl ester, Propionic ester, Ethyl propanoate, top fragrance |
Numbering system
CAS number:105-37-3
MDL number:MFCD00009308
EINECS number:203-291-4
RTECS number:UF3675000
BRN number:506287
PubChem number:24890343
Physical property data
1. Properties: water-white liquid with pineapple aroma. [1]
2. Melting point (℃): -73.9[2]
3. Boiling point (℃): 99.1[3]
4. Relative density (water = 1): 0.89[4]
5. Relative vapor Density (air=1): 3.52[5]
6. Saturated vapor pressure (kPa): 5.32 (27.2℃)[6]
7. Critical pressure (MPa): 3.362[7]
8. Octanol/water partition coefficient: 1.21~1.32[8]
9. Flash point (℃): 12.2 (CC) [9]
10. Ignition temperature (℃): 440 [10]
11. Explosion upper limit (%): 11.0[11]
12. Explosion lower limit (%) : 1.8[12]
13. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, ether, and propylene glycol. [13]
14. Viscosity (mPa·s, 15ºC): 0.89574
15. Heat of evaporation (KJ/mol): 34.24
16. Heat of fusion (KJ/mol): 12.56
17. Heat of combustion (KJ/mol, b.p.): 2892.7
18. Specific heat capacity (KJ/(kg ·K), 59.6ºC, constant pressure): 2.07
19. Conductivity (S/m, 17ºC): 8.3×10-4
20. Heat of formation (KJ/mol): 532.14
21. Relative density (25℃, 4℃): 0.8840
22. Refractive index at room temperature (n20): 1.3839
23. Refractive index at room temperature (n25): 1.3814
24. Critical density (g·cm– 3): 0.299
25. Critical volume (cm3·mol-1): 342
26. Critical compression factor: 0.260
27. Eccentricity factor: 0.394
28. Solubility parameter (J·cm-3)0.5 : 17.667
29. van der Waals area (cm2·mol-1): 9.140×109
30. van der Waals volume (cm3·mol-1): 63.000
31. Gas phase Standard heat of combustion (enthalpy) (kJ·mol-1): -2933.11
32. Gas phase standard claims heat (enthalpy) (kJ·mol-1 sup>): -463.59
33. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2893.99
34. Liquid Phase standard claims heat (enthalpy) (kJ·mol-1): -502.71
35. Liquid phase standard hot melt (J·mol-1·K-1):200.2
Toxicological data
1. Acute toxicity[14]
LD50: 8732mg/kg (rat��oral); 3500mg/kg (rabbit oral)
2. Irritation [15]
Rabbit Transdermal: 500mg (24h), moderate irritation.
Rabbit eye: 100mg, moderate irritation.
Ecological data
1. Ecotoxicity[16]
LC50: 56mg/L (48h) (rainbow trout); 45~170mg/L ( 48h) (Daphnia)
EC50: 811mg/L (15min) (Photobacterium, Microtox toxicity test)
IC50: 14mg/L (72h) (Scenedesmus quadradata)
2. Biodegradability No information yet
3. Non-biodegradability [17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 7d (theoretical).
When the pH value is 7 and 8, the hydrolysis half-life is 2.5a and 90d respectively (theoretical).
Molecular structure data
1. Molar refractive index: 26.98
2. Molar volume (cm3/mol): 114.5
3. Isotonic specific volume (90.2K ): 255.8
4. Surface tension (dyne/cm): 24.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 10.69
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 59.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the chemical properties of general esters.
2. Stability[18] Stable
3. Incompatible substances[19] Strong oxidants, alkalis, acids
4. Polymerization hazard[20] No polymerization
Storage method
Storage Precautions[21] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Mainly adopt esterification method. It is obtained by using propionic acid and ethanol as raw materials, carrying out esterification reaction in the presence of concentrated sulfuric acid catalyst, and then neutralizing, washing, drying and distilling.
2. It is produced by direct esterification of propionic acid and ethanol, followed by washing and fractionation.
Refining method: Contains water, propionic acid, ethanol and other impurities. During refining, neutralize with sodium carbonate solution, wash with water and calcium chloride solution respectively, dry with anhydrous potassium carbonate and then distill.
![]()
3. In the glass-lined reactor , add 38kg of industrial ethanol and 50kg of n-propionic acid, stir for 15 minutes, add 38kg of anhydrous calcium chloride, and heat to reflux under stirring for 10 hours. 80~85L of crude product was distilled. 80L of crude product is washed 3 to 4 times with 5% to 10% sodium carbonate solution under stirring. After the acidity is qualified, it is washed once with water and dried with 2 to 3Kg of industrial carbonic acid. Distill 50kg of the dried crude product in a stainless steel vessel and collect the 96-100°C fraction to obtain 40L of finished product.
The process reaction formula is:
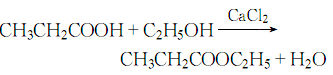
Purpose
1. Used as a solvent for various natural and synthetic resins and a solvent for paint. Also used in artificial flavors and organic synthesis. Mainly used for preparing fruity and floral flavors and as top flavoring agent.
2. Used to prepare bananas, pineapples, cream, raw pears, bubble gum, jackfruit, rum, apples, grapes, peaches, tropical fruits and other edible flavors. It can also be used for tobacco and wine flavors. middle. The usage amount in gum is 1100mg/kg; in baked goods 110mg/kg; in candies 78mg/kg; in cold drinks 29mg/kg; in puddings 10-15mg/kg; in soft drinks 7.7mg/kg.
3. Used as cellulose esters and ethers, the volume of various natural or synthetic resins; as a spice, it can be used to blend apple aroma, banana aroma, plum aroma, and as butter, Western wine and many other Food flavors. It can also be used as a high-end daily cosmetics fragrance. It is also used as an organic synthesis intermediate for the production of antimalarial drugs such as ethimethamine.
4. Can be used in baked goods, candies, and frozen dairy products.
5. Used as a solvent and also used in organic synthesis. [22]
