Ethylene diamine tetraacetic acid Ethylene diamine tetraacetic acid

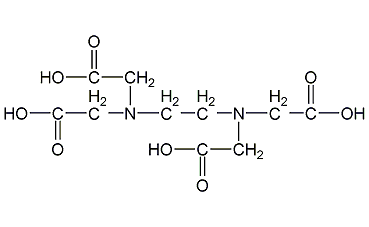
Structural formula
| Business number | 01AV |
|---|---|
| Molecular formula | C10H16N2O8 |
| Molecular weight | 292.24 |
| label |
Diaminoethylene tetraacetate, Acetic acid, Veronic acid, (Ethylenedinitrilo)tetraacetic acid, Edathamil, EDTA, polymerization initiator, Fiber processing aids, cosmetic additives, complexing agent, blood anticoagulants, Quantitative analysis reagents for heavy metals |
Numbering system
CAS number:60-00-4
MDL number:MFCD00003541
EINECS number:200-449-4
RTECS number:AH4025000
BRN number:1716295
PubChem number:24894713
Physical property data
1. Properties: white or milky white crystalline powder. There is a slight fragrance. Bitter taste.
2. Melting point (ºC): 240-241
3. Decomposition temperature (ºC): 250
4. Solubility: insoluble in ethanol and general Organic solvent, slightly soluble in cold water, soluble in aqueous solutions of sodium hydroxide, sodium carbonate and ammonia. Its alkali metal salt is soluble in water. Can be dissolved in more than 5% of inorganic acids, soluble in sodium hydroxide or sodium carbonate aqueous solution, and also soluble in ammonia and 160 parts of boiling water.
Toxicological data
Non-toxic and non-irritating. The LC50 of intraperitoneal injection in mice is 0.25g/kg; the LD50 of intraperitoneal injection in rats is 0.39g/kg. Slightly irritating to skin, eyes and mucous membranes.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 62.07
2. Molar volume (cm3/mol): 186.6
3. Isotonic specific volume (90.2K ): 568.5
4. Surface tension (dyne/cm): 86.1
5. Polarizability (10-24cm3): 24.60
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -5.9
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 10
4. Number of rotatable chemical bonds: 11
5. Number of tautomers: none
6. Topological molecule polar surface area 156
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 316
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. The number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Solubility in water is 0.5g/L (25℃). Decarboxylation occurs when heated to 150°C.
2. Avoid ingestion, inhalation and contact with skin and eyes.
3.Can form stable complexes with a variety of metal ions, and is a commonly used complexing agent for electroplating and electroless plating
Storage method
1. Store sealed in a cool, dry place.
2.Not suitable for mixed storage with alkaline chemicals
Synthesis method
1. In a 1L three-necked flask equipped with a stirrer and thermometer, add 125g chloroacetic acid, 150g ice and 130mL 30% sodium hydroxide solution, and then add 20g ethylenediamine while stirring. After incubation at 15°C for 1 hour, add 30% sodium hydroxide solution in batches of 10 mL each time. After each addition, wait until the phenolphthalein indicator is no longer alkaline before adding the next batch. The final reaction mixture should be alkaline. After keeping at room temperature for 12 hours, heat to 90°C and add activated carbon for decolorization. Filter, wash the filter residue with water, and the total volume after combining the washing liquid and filtrate is about 700 mL. Add concentrated hydrochloric acid to pH=3, crystals will begin to precipitate, filter out the crystals, and wash with water until there is no chloride ion. After drying, the product ethylenediaminetetraacetic acid is about 80g, with a yield of 90%. The reaction formula is as follows:

2.In ethylenediaminetetraacetic acid and water, add 30% sodium hydroxide solution with stirring, heat to complete reaction, add hydrochloric acid until the ph value is 4.5, concentrate, filter and cool Crystallize, wash and dry to obtain ethylenediaminetetraacetic acid.
3.In a 800l stainless steel reaction pot, add 100kg chloroacetic acid, 100kg ice and 135kg 30% sodium hydroxide solution. Add 18kg of ethylenediamine at 83-84℃ while stirring. After incubating at 15℃ for 1 hour, add 30% sodium hydroxide solution in batches of 10L each time. After each addition, wait until the phenolphthalein indicator is no longer alkaline before adding Next batch, the final reactants are alkaline. After keeping at room temperature for 12 hours, heat to 90°C, add activated carbon, filter, and wash the filter residue with water. Finally, the total volume of the solution is about 600L. Add concentrated hydrochloric acid to a pH of 3 to precipitate crystals. Filter and wash
until there is no chlorine radical. After drying, 64kg of EDTA was obtained, with a yield of 95%. Then wash with water to get the finished product. The process reaction formula is:
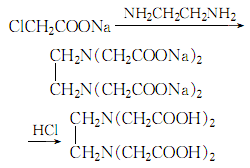
4. React ethylenediamine with formaldehyde and sodium cyanide. Mix 60% ethylenediamine aqueous solution, 30% sodium cyanide aqueous solution and sodium hydroxide Mix and keep mixing at 20°C for 0.5h. Then add formaldehyde aqueous solution dropwise. After the reaction, water was evaporated under reduced pressure. Then repeat the above operation, adding excess formaldehyde for the last time to allow the sodium cyanide to react completely. Adjust the pH to 1.2 with dilute acid. A white precipitate precipitated, filtered, washed with water, and dried at 110°C. Get the product. The reaction formula is as follows:

Purpose
1. Used as dyeing auxiliary in dye industry. In the rubber industry, it is used as a polymerization initiator for synthetic rubber. In the chemical fiber industry, it is used as fiber processing aid. Used in light industry to manufacture detergents. Used as cosmetic additives in the daily chemical industry. In the photosensitive industry, it is used as a bleaching and fixing solution for the processing of color photosensitive materials. Used in the pharmaceutical industry as a blood anticoagulant. Used as complexing agent in chemical production. Reagents and solvents for quantitative analysis of heavy metals.
2.Complexing reagent. Used as metal ion complexing agent and masking agent for separation, detergent, blood anticoagulant and agricultural chemical spray.
3.EDTA is a representative chelating agent, which is effective for calcium, magnesium , iron scale ions have complexing or chelating effect, good scale dissolving effect, little corrosiveness to metal facilities, no hydrogen embrittlement and intergranular corrosion, and can be used for non-stop cleaning in the circulating water system, but because of its high price, Shortcomings such as slow scale dissolution speed and inability to remove silicon scale at room temperature limit its application.
4.Used as analytical reagents, such as compounding agents and masking agents. It is also used in the preparation of detergents, blood anticoagulants, and agricultural sprays.
5.It can be used as a complexing agent in the electroplating solution and as a ph value regulator in the nickel plating solution. It is used as a brightener in sodium salt passivation solution to eliminate white spots on the titanium salt passivation film. The general dosage is 8g/L.
6.When used in hair dye cosmetics, when peroxide is mixed with dye composition, metal ions will catalyze the peroxide The decomposition of hair dye will affect the hair dyeing effect
The addition of EDTA can effectively chelate trace amounts of heavy metals and increase the antioxidant effect. The amount added in hair dye cosmetics is generally 0.2%.
7.Ethylenediaminetetraacetic acid is used as a complexing agent in cyanide-free electroplating. It is a commonly used reagent in assays and can also be used as a complexing agent. Used as an additive in some plating baths and chemical treatment solutions. Its disodium salt is a complexing agent for rapid electroless copper plating.
