Fenaminosulf Fenaminosulf
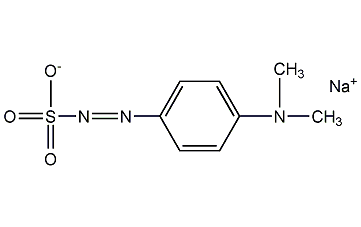

Structural formula
| Business number | 03T5 |
|---|---|
| Molecular formula | C8H10N3NaO3S |
| Molecular weight | 251.24 |
| label |
Sodium p-dimethylaminobenzene diazosulfonate, Sodium p-dimethylaminobenzene diazosulfonate, 4-Dimethylaminobenzenediazosulfonic acid sodium salt, Sodium-p-dimethylaminobenzenediazosulphonate, Fungicide |
Numbering system
CAS number:140-56-7
MDL number:MFCD00059900
EINECS number:205-419-4
RTECS number:CZ1750000
BRN number:4170376
PubChem number:24899174
Physical property data
1. Physical property data:
1. Properties: light yellow crystal
2. Melting point (℃): 200℃ (decomposition)
3. Solubility: Soluble in highly polar solvents, such as dimethylformamide, ethanol, etc., but insoluble in most organic solvents. Soluble in water, with a solubility of 2-3% at room temperature.
Toxicological data
2. Toxicological data:
1. Acute toxicity: Rat oral LD50: 60 mg/kg;
Rat skin LD50: >100 mg/kg;
Rat abdominal cavity LD50: 10300 UG/KG;
mice LD50: 140 mg/kg;
Mouse abdominal cavity LD50: 60 mg// kg;
Mouse intravenous LD50: 56 mg/kg;
Rabbit oral LD50: 156 mg/kg;
Rabbit intraperitoneal LD50: 10 mg/kg kg
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 93.5
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 318
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure.
Storage method
Store sealed in a dry and cool place.
Synthesis method
1. Prepared from N, N-dimethylaniline through nitrosation, reduction, diazotization and sulfonation. Dimethylaniline and a slight excess of sodium nitrite are nitrosated at 0-5°C. After adding nitrous acid, it is reduced with iron powder to prepare p-aminodimethylaniline. Then the iron powder and the generated ferric oxide are filtered off, and the filtrate is diazotized with sodium nitrite at 0-5°C. After adding sodium nitrite, sodium sulfite is used for sulfination. After the reaction reaches the end point, dixon will precipitate out. The content of the obtained Dikson original powder (dry product) was 98%. It can also be used as a product in the form of a paste with a content of 55%. To produce products of this specification, 310kg of dimethylaniline is consumed per ton. The wastewater from vanillin production contains p-aminodimethylaniline, which can be used to produce dimethonine, and can eliminate the two processes of nitrosation and reduction.
2. Prepared from N, N-dimethylaniline through nitrosation, reduction, diazotization and sulfonation. Dimethylaniline and a slight excess of nitrous acid are nitrosated at 0 to 5°C, and then reduced after adding sodium nitrite to prepare p-aminodimethylaniline. Then diazotization is carried out under conditions of 0 to 5, and sodium nitrite is added, and then sulfination is performed with sodium sulfite to prepare sodium disulfonate.
Since the wastewater from vanillin production contains p-aminodimethylaniline, using it for production can save the two steps of nitrosation and reduction. Vanillin wastewater is first neutralized with ammonia water to a pH value of 7 to 8, heated to about 40°C, and allowed to settle for 8 to 12 hours to remove coke residue. Use benzene to extract p-aminodimethylaniline in the neutralization solution. The extract is debenzenized at 130-150°C and 21.28kPa, and then distilled under reduced pressure at 2.66kPa to 130-150°C to obtain p-aminodimethyl Aniline boutique. After diazotization with sodium nitrite and sulfonation with sodium sulfite, sodium dimethosulfonate is prepared.
Purpose
1. Protective fungicide, with certain systemic penetration, is mainly used for seed treatment and soil treatment of vegetables, cotton, tobacco, rice and other crops, and has a certain therapeutic effect. To prevent cotton blight and anthracnose, use 250-500g of 70% wettable powder, add 5kg of fine soil and mix with 50kg of cotton seeds for sowing; to prevent and control seedling rot in rice, use 7.5-15g of 70% wettable powder/100m2 and add 7.5kg of water; evenly Spray seedlings; to control cabbage soft rot, tomato blight, anthracnose, melon wilt and anthracnose, use 70% wettable powder 27.6~55.3g/100m2 to spray with water.
2. This product is a seedling fungicide that has special effects on diseases caused by Pythium and Hypomyces. It is also effective against some fungal diseases. This product has a systemic effect and can be absorbed by the roots and leaves. It is imported into other parts from the sieve tube part and xylem of the plant, and can maintain the medicinal effect for a long time in the plant. It can prevent and control rice blight, black root disease, seedling rot, sorghum head smut, smut, corn leaf spot, cotton seedling root rot, anthracnose, blight, etc.
3. It is a seedling fungicide that can prevent and control rice seedling blight, sorghum head smut, corn leaf spot, cotton seedling root rot, anthracnose, etc.
