Hexachlorocyclopentadiene Hexachlorocyclopentadiene
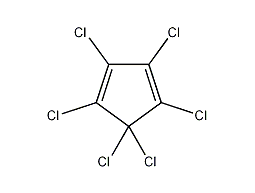
Structural formula
| Business number | 01LS |
|---|---|
| Molecular formula | C5Cl6 |
| Molecular weight | 270 |
| label |
Ester cyclic compounds and their derivatives |
Numbering system
CAS number:77-47-4
MDL number:MFCD00001352
EINECS number:201-029-3
RTECS number:GY1225000
BRN number:None
PubChem number:24895723
Physical property data
1. Properties: Yellow to amber oily liquid with pungent odor and non-flammable.
2. Density (g/mL, 25/4℃): 1.7019
3. Relative vapor density (g/mL, air=1): 9.42
4. Melting point (ºC): -9.6
5. Boiling point (ºC, normal pressure): 239
6. Boiling point (ºC, 0.133~1.173kPa): 68 ~70
7. Refractive index (20ºC): 1.5644
8. Flash point (ºC): Uncertain
9. Specific rotation (º) : Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): 0.012kPa
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion Upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Insoluble in water, soluble in Most organic solvents such as ether and carbon tetrachloride
Toxicological data
This product is toxic. Contact with the skin can cause poisoning through the blood, and can be harmful to the liver and other organs. This product with a purity of 93.3% has an oral lethal dose of 420 to 620 mg/kg body weight for rats and rabbits, and a skin absorption lethal dose of 430 to 610 mg/kg body weight. Absorption through the skin causes poisoning and is harmful to the liver and other organs. Corrosive. The maximum time rats can survive in an atmosphere of 250mg/m3 is 0.25h.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 51.06
2. Molar volume (cm3/mol): 148.8
3. Isotonic specific volume (90.2K ): 390.7
4. Surface tension (dyne/cm): 47.4
5. Polarizability (10-24cm3):20.24
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): None
2. Hydrogen bond donor�Number of bonds: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Tautomers Number: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 234
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain Number of atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15 .Number of covalent bond units: 1
Properties and stability
1. Non-flammable. Corrosive. This product is toxic and should be washed immediately with alkaline solution after contact with the skin.
2.This product is toxic. It can be absorbed into the blood through the skin and cause poisoning. It can be harmful to the liver and other organs. For acute poisoning, the maximum time that rats can survive in an atmosphere of 250 mg/m3 is 0.25 hours. The oral LD5 of hexachlorocyclopentadiene with a purity of 93.3% for rats and rabbits is 420 to 620 mg. /kg body weight. After contact with skin, wash immediately with alkaline solution. The equipment should be sealed and the workshop should be well ventilated. Operators should wear protective equipment.
Storage method
This product is kept sealed and leakage is strictly prohibited. Use corrosion-resistant equipment for storage, such as acid-resistant ceramic altars, etc. Store and transport according to regulations on flammable and toxic substances.
This product is stored in corrosion-resistant equipment, such as acid-resistant ceramic jars. Store and transport according to regulations on flammable and toxic substances.
Synthesis method
It is obtained by chlorination of cyclopentadiene in two steps. The first step of chlorination is carried out at 90°C. The weight ratio of chlorine to cyclopentadiene is 1: (0.24-0.34). The reaction generates chlorinated cyclopentane containing 4-6 chlorines. The second step of chlorination is carried out at high temperature, the reaction temperature is 500-510°C, and the molar ratio of polychlorinated cyclopentane and chlorine is 1: (6-8). 
In addition, petroleum pentane is used as raw material, which is photochlorinated and Hexachlorocyclopentadiene is synthesized by high-temperature chlorination with a yield of about 70%.
Purpose
This product is used as a reactive flame retardant in the manufacture of flame retardant polyester resin and flame retardant polyurethane foam. It is also used in the manufacture of chlorine-containing flame retardants such as perfluoropentacyclodecane, Hite anhydride, and Declon. This product is also used to prepare organochlorine pesticides, aldrin, chlordane, chlordane, etc.
