L-Adrenaline L-Adrenaline
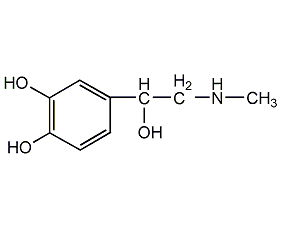
Structural formula
| Business number | 014U |
|---|---|
| Molecular formula | C9H13NO3 |
| Molecular weight | 183.2 |
| label |
(R)-Adrenaline, 1-(3,4-dihydroxyphenyl)-2-methylaminoethanol, 3,4-Dihydroxy-α-(methylaminomethyl)benzyl alcohol L-epinephrine, L(-)-adrenaline, Renal parabase, (R)-(−)-3,4-Dihydroxy-α-(methylaminomethyl)benzyl alcohol, Epinephrine, Adnephrin, Epifrina, Suprarenine, Sus-Phrine, Tonogen, Vaponefrin, Enzymes·Proteins·Peptides |
Numbering system
CAS number:51-43-4
MDL number:MFCD00002204
EINECS number:200-098-7
RTECS number:DO2625000
BRN number:2368277
PubChem number:24894545
Physical property data
1. Properties: White crystalline powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 211-212
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º, C= 2, in 0.6mol/L hydrochloric acid): -50~-53.5
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in mineral acid and alkali hydroxide solution, slightly soluble in water, insoluble in alcohol, ether, acetone, and chloroform. Odorless and bitter. The color gradually becomes darker when exposed to air and light.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 49.33
2. Molar volume (cm3/mol): 142.6
3. Isotonic specific volume (90.2K ): 397.0
4. Surface tension (dyne/cm): 59.9
5. Polarizability (10-24cm3): 19.55
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 10
6. Topological molecule polar surface area 72.7
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 154
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Seal with nitrogen and store in a cool, dark place.
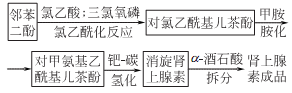
Synthesis method
1.3,4-Dihydroxy-α-methylaminoacetophenone (i.e. renal ketone) is formulated into hydrochloride, and hydrogenation reaction is carried out under palladium catalysis. The hydrogenation temperature is 30-35α and the pressure is 0.05-0.1MPa. The obtained racemic epinephrine is separated with tartaric acid and neutralized with ammonium hydroxide to obtain L-adrenaline.
2.Extraction, isolation and purification from the adrenal medulla of domestic animals.
3.Artificial synthesis

Purpose
1. Non-steroidal hormone drugs are also used as intermediates for the hemostatic drug Anluoxue. Mainly used for the rescue of anaphylactic shock, bronchial asthma and cardiac arrest
2.Adrenoceptor agonist. Used for relieving bronchial asthma, rescuing anaphylactic shock, compatibility with local anesthetics and local hemostasis, urticaria, hay fever, serum reactions and other allergic diseases, glaucoma, etc.
