L-carnitine
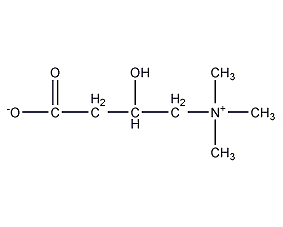

Structural formula
| Business number | 05KM |
|---|---|
| Molecular formula | C7H15NO3 |
| Molecular weight | 161.20 |
| label |
(R)-3-Carboxy-2-hydroxy-N,N,N-trimethylpropylammonium hydroxide inner salt, L-carnitine, Carnitine, Hydroxide-N,N,N-trimethyl-4-amino-3-hydroxybutyric acid inner salt, L-Carnitine inner salt, 3-Carboxy-N,N,N-trimethyl-2-propen-1-aminium chloride, L(-)-Carnitine, Nutrition supplements |
Numbering system
CAS number:541-15-1
MDL number:MFCD00038747
EINECS number:208-768-0
RTECS number:BP2980000
BRN number:4292315
PubChem ID:None
Physical property data
1. Appearance: white crystal or transparent powder
2. Density (g/ cm3, 25/4℃): Undetermined
3. Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC): 197-212
5 . Boiling point (ºC, normal pressure): Not determined
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: -32°
8. Flash point (ºC): Not determined
9. Specific rotation (º): -31°
10. Autoignition point or ignition temperature (ºC): Not determined Determined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 55.1ºC): Undetermined
13. Heat of combustion (KJ/mol): 39.8
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: 4.47
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (% ,V/V): Undetermined
19. Solubility: Dissolved in water
Toxicological data
Acute toxicity: subcutaneous LD50 in mice: 9 gm/kg, tearing of eyes, convulsions or epilepsy, structural or functional changes in salivary glands in the gastrointestinal tract;
Dogs via unknown route LD50: 7 gm /kg, no details except lethal dose;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.2
2.�Number of �� bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Interconversion Number of isomers: None
6. Topological molecule polar surface area 60.4
7. Number of heavy atoms: 11
8. Surface charge: 0
p>
9. Complexity: 134
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12 .The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. The uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Store at room temperature
Synthesis method
Extraction method
L-carnitine naturally exists in various meats and dairy products, so it can be directly extracted from beef and milk containing L-carnitine. According to literature reports, 0.6g of crystalline carnitine can be extracted from 450g of beef extract, and 100g of lactose powder containing 2% L-carnitine can be extracted from 56kg of milk. However, the extraction method is more expensive and is not economically reasonable.
Microbial fermentation method
Research shows that L-carnitine also exists in many microorganisms. L-carnitine can be accumulated by using liquid deep culture or solid fermentation of yeast, Aspergillus, Penicillium, Rhizopus and other microorganisms. However, due to the complexity of strain screening, the current fermentation level is still relatively low. According to reports, using 2% DL-carnitine as raw material and fermenting at 25°C for 44 hours, the accumulated L-carnitine was 0.4%.
Synthesis method
There was a patent report on the synthesis of DL-carnitine abroad in 1953, and industrial production began in the 1960s. In China, it was also produced and used as a stomach medicine in 1982. Starting directly from DL-carnitine, use camphoric acid, N-acetyl-D-glutamic acid or ethylbenzoyl-L-(+) tartaric acid as the resolving agent to perform chemical separation to obtain L-carnitine. However, racemization of oral carnitine is difficult and cannot be recycled. Industrial production still needs a breakthrough.
Enzymatic transformation
This is the most studied and promising method. DL-carnitine obtained through chemical synthesis can be first acetylated to produce amides or nitriles, and then selectively hydrolyzed and separated using enzymes derived from microorganisms. For example, Zhongshan Qing and others use the amidase of microorganisms such as Pseudomonas to selectively hydrolyze DL-carnitine amide or carnitine nitrile, and can produce L-carnitine with an optical purity of more than 99%. In addition, L-carnitine can also be prepared by enzymatic conversion of β-dehydrocarnitine, enzymatic hydrolysis of trans-croton betaine, and enzymatic hydroxylation of γ-butyl betaine.
Currently, only Switzerland, Italy, Japan and other countries produce it internationally. The Jiangsu Provincial Institute of Microbiology in my country is also conducting research on enzymatic conversion.
3-Chloro-2-hydroxypropyltrimethylamine chloride is prepared by reacting epichlorohydrin and trimethylamine to form a quaternary ammonium salt, and then After cyanation, it becomes 3-cyano-2-hydroxypropyltrimethylamine chloride, and then hydrolyzed with concentrated hydrochloric acid to obtain carnitine hydrochloride. Or use γ-ethyl bromoacetoacetate as raw material, first reduce it to ethyl γ-bromo-β-hydroxybutyrate with sodium tetrahydroborate, and then combine it with trimethylamine to form a quaternary ammonium salt, namely trimethylammonium bromide-β- Ethyl hydroxybutyrate and finally hydrolyzed carnitine hydrochloride with hydrochloric acid. It can also be extracted directly from beef and milk containing L-carnitine.
Purpose
1. It can promote fatty acid oxidation in mitochondria and achieve other biochemical functions. These functions include acetyl buffering and maintaining sufficient concentration of coenzyme A in mitochondria under anaerobic energy production, stimulating the circulation of tricarboxylic acid and muscle endurance. Exercise stimulates ATP export from mitochondria. For healthy growth of animals.
2.It is used to strengthen soy-based baby food and promote the absorption and utilization of fat. It is also a high-quality active substance for weight loss. Our country stipulates that it can be used in biscuits, bread and milk drinks, and the usage amount is 600~3000 mg/kg; the usage amount in solid drinks, liquids and capsules is 250~600mg/kg; the usage amount in milk powder is 300~400mg/kg ; The amount used in baby food is 70 to 90 mg/kg (based on L-carnitine, 1g tartrate is equivalent to 0.68gL -carnitine).
