Methane
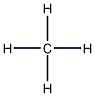
Structural formula
| Business number | 01HK |
|---|---|
| Molecular formula | CH4 |
| Molecular weight | 16.04 |
| label |
methyl hydride, liquefied methane, biogas, natural gas, Carbone, Firedamp, Firedamp, Methyl hydride, Marsh gas, aliphatic hydrocarbons, Electronic specialty gas raw materials and intermediates |
Numbering system
CAS number:74-82-8
MDL number:MFCD00008279
EINECS number:200-812-7
RTECS number:PA1490000
BRN number:1718732
PubChem ID:None
Physical property data
1. Properties: Colorless and odorless gas. [1]
2. Melting point (℃): -182.6[2]
3. Boiling point (℃): -161.4[3]
4. Relative density (water=1): 0.42 (-164℃)[4]
5. Relative vapor density (air=1): 0.6[5]
6. Saturated vapor pressure (kPa): 53.32 (-168.8℃)[ 6]
7. Heat of combustion (kJ/mol): -890.8[7]
8. Critical temperature (℃): -82.25[8]
9. Critical pressure (MPa): 4.59[9]
10. Octanol/ Water partition coefficient: 1.09[10]
11. Flash point (℃): -218[11]
12 .Ignition temperature (℃): 537[12]
13. Explosion upper limit (%): 15[13]
14. Lower explosion limit (%): 5[14]
15. Solubility: slightly soluble in water, soluble in ethanol, ether, benzene, toluene, etc. [15]
16. Lennard-Jones parameter (A): 3.7314
17. Lennard-Jones parameter (K): 151.16
18. Solubility parameter (J·cm-3)0.5: 11.618
19. van der Waals area (cm 2·mol-1): 2.880×109
20. van der Waals volume (cm3 sup>·mol-1): 17.050
21. Critical density (g·cm-3): 0.1627
22. Critical volume (cm3·mol-1): 98.60
23. Critical compression factor: 0.286
24 .Eccentricity factor: 0.011
25. Gas phase standard entropy (J·mol-1·K-1): 186.38
26. Gas phase standard free energy of formation (kJ·mol-1): -50.5
27. Gas phase standard hot melt (J·mol-1·K-1): 35.69
28. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -880.69
29. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -74.48
Toxicological data
1. Acute toxicity[16] LC50: 50% (mouse inhalation, 2h)
Ecological data
1. Health��Toxicity No data available
2. Biodegradability No data available
3. Non-biodegradability[17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 6a (theoretical).
4. Other harmful effects[18] Greenhouse gases. Special attention should be paid to contamination of surface water, soil, atmosphere and drinking water.
Molecular structure data
1. Molar refractive index: not available
2. Molar volume (cm3/mol): not available
3. etc. Zhang specific volume (90.2K): None available
4. Surface tension (dyne/cm): None available
5. Dielectric constant: None available
6. Polarizability (10-24cm3): None available
7. Single isotope mass: 16.0313 Da
8. Nominal mass: 16 Da
9. Average mass: 16.0425 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 1
8. Surface charge: 0
9. Complexity: 0
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Non-toxic. Exposure to methane is dangerous because it can displace oxygen and cause suffocation. Short-term exposure to methane can cause dizziness, difficulty breathing, bluish skin and loss of consciousness due to reduced oxygen available for breathing. Contact of skin and eyes with liquid methane can cause frostbite, and inhalation of liquid methane can cause frostbite in the mouth and throat. Pay attention to ventilation and prevent air leakage.
2. Rat inhalation LD50: 400×10-6. Methane can be considered a non-toxic gas but is an asphyxiant at high concentrations. When the methane concentration in the air reaches 10%, the eyes and forehead feel pressured. This feeling can disappear after breathing fresh air. At the highest concentration, symptoms of suffocation begin to appear, including rapid breathing, fatigue, nausea, vomiting, leading to loss of consciousness and death due to lack of oxygen.
3. Stability[19] Stable
4. Incompatible substances[20] Strong oxidants, strong acids, strong bases, halogens
5. Polymerization hazards[21] No polymerization
Storage method
Storage Precautions[22] This product in steel bottles should be stored in a cool, ventilated warehouse dedicated to flammable gases. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with leakage emergency response equipment.
Synthesis method
This product is the simplest organic compound and is widely distributed in nature. It is the main component of natural gas, coal bed methane and biogas and can be obtained after separation.
1. Separation from natural gas Natural gas contains 80% to 99% methane (φ). After cleaning the dry natural gas, use the wet natural gas to separate ethane using condensation, absorption and adsorption methods. Use it after the above light hydrocarbons.
2. Separation from oil field gas. Natural gas escapes from oil wells during oil extraction. Dry gas contains 80% to 85% methane (φ); wet gas contains 10% methane (φ). Under pressure and condensation, it can be liquefied and used as chemical raw materials.
3. Separation from refinery gas: The petroleum processing gas in each refinery contains 20% to 50% methane. When ethylene and propylene are separated from petroleum processing gases by absorption distillation and condensation distillation, methane, hydrogen or pure methane can be produced by-product.
4. Separation from coke oven gas: Coke oven gas contains about 20% to 30% methane, and retort gas contains about 40% to 60% methane. Methane is produced as a by-product when hydrogen is separated from coke oven gas using a cryogenic method.
5. Use the by-product methane (containing more than 98% CH4) of the natural gas helium extraction unit as raw material, pass through one or two low-temperature methane distillation towers to remove nitrogen and oxygen impurities, and then remove C2 through the adsorber From the above hydrocarbons, a high-purity methane product with a purity of more than 99.99% can be obtained. Or use the tail gas of the ethylene unit as the raw material gas, first remove water, carbon dioxide and hydrocarbon impurities above C2 through the adsorber, and then introduce it into the intermittent distillation tower for distillation. When the total impurity concentration index in the gas discharged from the top of the tower reaches the requirement, the distillation can be stopped, and high-purity methane with a purity of more than 99.995% can be produced.
Purpose
1. In addition to being used as fuel, it is widely used in the synthesis of ammonia, urea and carbon black. It can also be used to produce methanol, hydrogen, acetylene, ethylene, formaldehyde, carbon disulfide, nitromethane, hydrocyanic acid and 1,4-butadiene. Alcohol etc. Chlorination of methane can produce one, two, three chloromethane and carbon tetrachloride.
2. High-purity methane can be used in the manufacture of amorphous silicon solar cells and as an auxiliary gas for large-scale integrated circuit dry etching or plasma etching gas. It also produces ammonia, carbon black, and methylCompounds, carbon disulfide, hydrocyanic acid, etc.
3. Industrially, it is mainly used to produce acetylene or to produce hydrogen, synthetic ammonia and raw materials for organic synthesis through conversion.
4. Used as fuel and in the manufacture of carbon black, hydrogen, acetylene, formaldehyde, etc. [23]
extended-reading:https://www.newtopchem.com/archives/40512extended-reading:https://www.cyclohexylamine.net/methylcyclohexane-cas108-87-2/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-4.jpgextended-reading:https://www.bdmaee.net/efficient-trimerization-catalyst-for-aliphatic-and-alicyclic-isocyanates/extended-reading:https://www.bdmaee.net/fascat4233-catalyst-butyl-tin-mercaptan-arkema-pmc/extended-reading:https://www.newtopchem.com/archives/1905extended-reading:https://www.newtopchem.com/archives/44641extended-reading:https://www.newtopchem.com/archives/558extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/10.jpgextended-reading:https://www.bdmaee.net/di-n-octyltin-oxide/
