n-Decane Decane
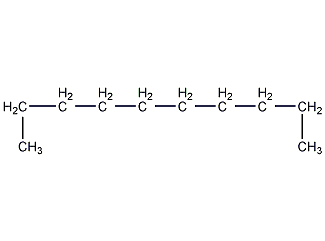
Structural formula
| Business number | 03HX |
|---|---|
| Molecular formula | C10H22 |
| Molecular weight | 142.28 |
| label |
n-Decane, linear compound |
Numbering system
CAS number:124-18-5
MDL number:MFCD00008954
EINECS number:204-686-4
RTECS number:HD6550000
BRN number:1696981
PubChem number:24869116
Physical property data
1. Properties: colorless and transparent liquid[1]
2. Melting point (℃): -29.7[2]
3. Boiling point (℃): 174.2[3]
4. Relative density (water=1): 0.73[4]
5. Relative vapor density (air=1): 4.9[5]
6. Saturated vapor pressure (kPa): 0.17 (25℃) [6]
7. Heat of combustion (kJ/mol): -6778.29[7]
8. Critical temperature (℃): 344.6[8]
9. Critical pressure (MPa): 2.11[9]
10. Octanol/water partition coefficient: 5.01[10]
11. Flash point (℃): 46 (CC)[11]
12. Ignition temperature (℃): 210[12]
13. Explosion upper limit (%): 5.4[13]
14. Lower explosion limit (%): 0.8[14]
15. Solubility: insoluble in water, miscible in ethanol and ether. [15]
16. Critical density (g·cm-3): 0.228
17. Critical volume (cm 3·mol-1): 624
18. Critical compression factor: 0.256
19. Eccentricity factor: 0.484
20. Solubility parameter (J·cm-3)0.5: 15.538
21. van der Waals area (cm 2·mol-1): 1.504×1010
22. van der Waals volume (cm 3·mol-1): 109.180
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -6829.63
24. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -249.58
25. Gas phase standard entropy (J· mol-1·K-1): 545.85
26. Gas phase standard formation free energy (kJ·mol-1): 33.03
27. Gas phase standard hot melt (J·mol-1·K-1): 233.0
28. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -6788.29
29. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -300.91
30. Liquid phase standard entropy (J·mol-1·K-1) : 425.93
31. Liquid phase standard free energy of formation (kJ·mol-1): 17.44
32. Liquid phase standard hot melt (J· mol-1·K-1): 314.5
Toxicological data
1. Acute toxicity:
Inhalation LC50 in mice: 72300mg/m3/2H; intravenous injection of LDLO in mice: 912mg/kg;
2. Oncogenicity: mouse skin TDLO: 25gm/kg/52W-I;
3. Acute toxicity[16] LC50: 72300mg/m3 (mouse inhalation, 2h)
4. Irritation No information available
5. Subacute and chronic toxicity[17] Rat inhaled 540ppm, 18h a day, 7d a week, lasting 57d, has a significant impact on body weight, and the total number of white blood cells decreases significantly, but there are no bone marrow or other obvious pathological changes. After inhaling a large amount of 500mg/m3 for 30 days and nights, the activities of catalase, cholinesterase, and dihydroxyribonuclease in the blood were reduced, and the sulfhydryl content was also reduced, which became more significant with the time of exposure. .
6. Carcinogenicity[18] The lowest transdermal toxic dose in mice (TDLo): 25g/kg ( 52 weeks, intermittent), positive for tumorigenesis.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[19] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 33.2h (theoretical ).
3. Bioconcentration[20] BCF: 143.8 (theoretical)
4. Other harmful effects[21] This substance may be harmful to the environment , special attention should be paid to the pollution of surface water, soil, atmosphere and drinking water.
Molecular structure data
1. Molar refractive index: 48.37
2. Molar volume (cm3/mol): 193.6
3. Isotonic specific volume (90.2K ): 430.0
4. Surface tension (dyne/cm): 24.3
5. Polarizability (10-24cm3):19.17
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 40
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[22] Stable
2. Incompatible substances [23] Strong oxidants, strong acids, strong bases, halogens
3. Polymerization hazards[24] No aggregation
Storage method
Storage Precautions[25] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:
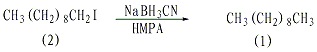
In a dry reaction bottle equipped with a stirrer, thermometer, and reflux condenser (installed with a calcium chloride drying tube), add 25 mL of hexamethylphosphoramide (HMPA), 1-iododecane (2 )2.7g (0.01mol), sodium cyanoborohydride 0.943g (0.015mol). React at 70°C for 2 hours with stirring. After cooling, add 25 mL of water and extract three times with diethyl ether. Combine the ether extracts, wash twice with water, dry over anhydrous magnesium sulfate, and distill under reduced pressure. Collect the 68-79°C/1.86KPa fraction to obtain 1.25-1.3g of n-decane, with a yield of 88%-90%. [27]
2. Preparation method:
![]()
Use 23g (1.0mol) sodium metal and 75.5g (62mL, 0.5mol) 1-bromopentane (2) or 99g (65.5mL, 0.5mol) 1 – Iodopentane According to the above operation method for preparing n-octane, collect the fraction between 171 and 174°C to obtain about 28g of n-decane (1), with a yield of 79%. [28]
Purpose
1. Used as a solvent, in organic synthesis, and in fuel research. [26]
