Nicotinic acid Nicotinic acid

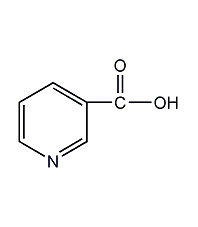
Structural formula
| Business number | 01AM |
|---|---|
| Molecular formula | C6H5NO2 |
| Molecular weight | 123.11 |
| label |
3-Picolinic acid, Pyridine-3-carboxylic acid, Vitamin B3, Pellagra preventive factor, Nutrition supplements, Heterocyclic compounds |
Numbering system
CAS number:59-67-6
MDL number:MFCD00006391
EINECS number:200-441-0
RTECS number:QT0525000
BRN number:109591
PubChem number:24897668
Physical property data
1. Properties: Colorless needle-like crystals. Odorless. The taste is slightly sour. It does not deliquesce in the air and is stable. Can sublimate without decomposing.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 236
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: 1g product dissolves in 60ml of water, easily soluble in Boiling water, boiling ethanol, alkali hydroxide and alkali carbonate solutions are soluble in propylene glycol and insoluble in ether.
Toxicological data
Acute toxicity:
Oral LD50 3720mg/kg(mus)
7mg/kg(rat)
Eye irritation moderate unknown mg(rbt)
Main irritant effects:
On skin: May cause inflammation.
On eyes: Irritation effects
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard class 1 (German Regulation) (self-assessment via list) This substance is slightly hazardous to water.��.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 31.27
2. Molar volume (cm3/mol): 95.1
3. Isotonic specific volume (90.2K): 263.5
4. Surface tension (dyne/cm): 58.7
5. Polarizability (10-24cm3): 12.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 50.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 114
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored away from light.
Synthesis method
Niacin was originally obtained by oxidizing nicotine. In industry, it is mostly alkylpyridine, such as 3-methylpyridine, 2-methyl-5-ethyl It is prepared using pyridine (MEP) and quinoline as raw materials. From the perspective of synthesis methods, it is generally divided into reagent oxidation method, ammonia oxidation method, gas phase oxidation method, electrolytic oxidation method and other methods.
1. Nitric acid oxidation method: MEP, 3-methylpyridine or quinoline are liquid-phase oxidized with nitric acid to produce nicotinic acid.
2. Vapor phase oxidation method: This method is characterized by using air (or oxygen-rich air) as the oxidant, and under the action of a catalyst, 3-methylpyridine is oxidized to produce nicotinic acid. Anhui Chaoyang Chemical Factory adopted this method and put it into production. Since this method uses air as the oxidant, the catalyst can be recycled for a long time, and the oxidation is completed in one step. It is less polluting than the ammonia oxidation method. It is a low-cost, high-efficiency and promising method.
3. Potassium permanganate oxidation method uses 3-methylpyridine as raw material and oxidizes it with potassium permanganate to obtain nicotinic acid. The reaction formula is as follows:![]()
4. Ammonia oxidation method: 3-methylpyridine (or MEP) is catalytically oxidized in the gas phase with air and ammonia to obtain nicotinonitrile; nicotinonitrile is further hydrolyzed to obtain nicotinamide or nicotinic acid. The reaction formula is as follows:
Purpose
Nutrients, preparation of tissue culture media. This product is a vitamin drug, collectively known as vitamin PP together with nicotinamide. It is used to fight pellagra. It can also be used as a blood dilator and is widely used as an additive in food and feed. As a pharmaceutical intermediate, it is used in the production of isoniazid, nicotinamide, nicotine and inositol nicotinate, etc.
