Norbornene dicarboxylic anhydride 5-Norbornene-2,3-dicarboxylic Anhydride
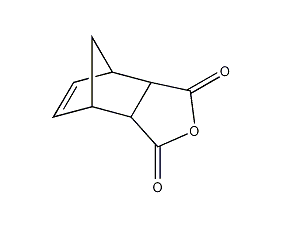
Structural formula
| Business number | 03M4 |
|---|---|
| Molecular formula | C9H8O3 |
| Molecular weight | 164.16 |
| label |
3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride, Endomethylenetetrahydrophthalic anhydride, 3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride, 5-Nonene-2,3-dicarboxylic acid anhydride, 5-Norbornene-2,3-dicarboxylic anhydride, cis-endo-5-Norbornene-2,3-dicarboxylic anhydride, cis-endo-Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride, Hardener, plasticizer, pesticides, Rubber vulcanization regulator, surfactant, Textile finishing penetrant |
Numbering system
CAS number:129-64-6
MDL number:MFCD00151106
EINECS number:204-957-7
RTECS number:None
BRN number:83657
PubChem number:24854879
Physical property data
1. Character: white columnar crystal
2. Density (g/mL, 25/4℃): 1.417
3. Melting point (℃): 164~165
4. Solubility: Soluble in benzene, toluene, acetone, carbon tetrachloride, chloroform, ethanol, ethyl acetate, slightly soluble in petroleum ether.
5. Stability: Deliquescent and sublimates when heated. Reacts with water to generate the corresponding acid. Irritating to skin and mucous membranes.
Toxicological data
Irritating to skin and mucous membranes
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 99.55
2. Molar volume (cm3/mol): 333.8
3. Isotonic specific volume (90.2K ): 813.4
4. Surface tension (dyne/cm): 35.2
5. Polarizability (10-24cm3): 39.46
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 43.4
7. Number of heavy atoms: 12
8, Surface charge: 0
9. Complexity: 277
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 4
12. The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. Uncertain chemical bond stereocenters Quantity: 0
15. Quantity of covalent bond units: 1
Properties and stability
It is deliquescent and sublimates when heated. It has an irritating effect on the skin and mucous membranes. Operators should wear protective equipment.
Storage method
Store in a cool and ventilated warehouse, away from fire and heat sources and avoid sunlight.
Synthesis method
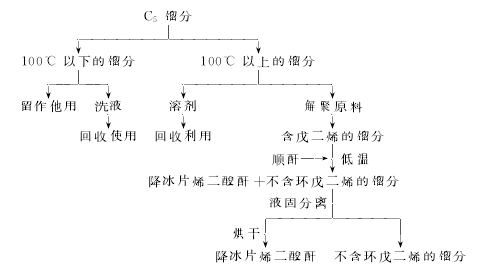
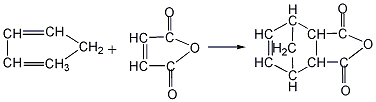
(1) Add maleic anhydride, cyclopentadiene and solvent benzene into the reaction kettle, stir and dissolve to carry out DielsAlder reaction, the reaction temperature is 20~60℃, the reaction product The yield of endomethine tetrahydrophthalic anhydride can reach 100%. At the end of the reaction, the solvent benzene is evaporated by heating and recycled for recycling. The crude anhydride can be refined by sublimation to obtain the finished product.
(2)Norbornene anhydride can be prepared by Diels Alder reaction between the C5 fraction, a by-product in the process of cracking petroleum hydrocarbons to produce ethylene, and maleic anhydride. Divide the C5 fraction raw material into three fractions: ~50°C, ~100°C, and >100°C. Select the 100°C fraction as the washing liquid, the >100°C fraction as the reaction solvent without distillation, and the >100°C fraction undergoes high-temperature depolymerization at 170°C. As the reaction raw material, the synthesis conditions are: maleic anhydride: cyclopentadiene = 1:1.2 (molar ratio), temperature 40°C, reaction time 3 hours, and the product yield is about 85% (based on maleic anhydride). The process flow is as follows:
(3)Add a certain amount of maleic anhydride (MA) and solvent into a 250ml four-necked flask equipped with a stirrer, reflux condenser and thermometer (The dosage is 1.5 mL per gram of maleic anhydride), stir thoroughly, heat to a certain temperature, drop a certain amount of cyclopentadiene (the molar ratio of maleic anhydride to cyclopentadiene is 1:1.3), and heat at 35°C The reaction was stopped after 1.5 hours of incubation. The reaction solution was frozen and crystallized, filtered, and vacuum dried to obtain the target product NA. The product yield is 90%, the melting point is 158-162°C, and the purity is 96.5%.
Purpose
Mainly used as a curing agent for epoxy resin, suitable for casting, lamination, powder molding, etc. The general dosage is 75-95℃, curing conditions: 100℃/2 hours + 150℃/5h. The cured product has excellent weather resistance, heat resistance and electrical properties. This product can also be used as a raw material for polyester resin, alkyd resin, plasticizer, stability and insecticide. Some derivatives and their uses are as follows: Diallyl norbornedioate is used as a heat-resistant cross-linking agent for unsaturated polyesters. 3,6-Endomethylene-1,2,3,6-tetrahydrophthalic acid epoxidized didecyl ester (EDET). This is an excellent epoxy stabilizer for polyvinyl chloride, which can be obtained by decanol esterification and epoxidation. Norbornene diic anhydride can be obtained through N-hydroxynorbornene dicarboximide to produce O,O-dimethyl-3,6-endomethylene-1,2,3,6-tetrahydro-phthalate Dicarboximide oxyphosphorothioate. This is a very effective pesticide. This product is also used as a modifier for urea-formaldehyde resin, melamine resin, rosin, etc., a vulcanization regulator for rubber, a plasticizer for resin, a surfactant, and a penetrating agent for textile finishing. This product is irritating to skin and mucous membranes.
