o-Methoxyacetanilide o-Acetanisidide
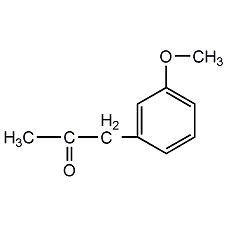

Structural formula
| Business number | 0262 |
|---|---|
| Molecular formula | C9H11NO2 |
| Molecular weight | 165.19 |
| label |
2-acetamidoanisole, Acetaminophen, o-Methoxyacetanilide, o-Acetamidoanisole, N-Acetylanisidine, N-Acetyl-o-anisidine, N-Acetyl-ortho-anisidine, o-Methoxyacetanilide, o-Acetanisidine |
Numbering system
CAS number:93-26-5
MDL number:MFCD00026117
EINECS number:202-233-5
RTECS number:AE8280000
BRN number:None
PubChem number:24872018
Physical property data
1. Properties: White needle-like crystals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 87-88
5. Boiling point (ºC, normal pressure): 303-305
6. Boiling point (ºC, 5.2kPa) : Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12 . Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
p>
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot water and ethanol, soluble in Ether and other organic solvents. The solubility in water, acetic acid, ether and ethanol is above 10%. Slightly soluble in cold water.
Toxicological data
The median lethal dose (mice, oral) 940 mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 47.20
2. Molar volume (cm3/mol): 146.5
3. Isotonic specific volume (90.2K ): 367.6
4. Surface tension (dyne/cm): 39.6
5. Polarizability (10-24cm3): 18.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 38.3
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 159
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Store in a sealed, cool, dry and dark place.
Synthesis method
Obtained from the reaction of anthranilic ether and acetic anhydride. Put the measured amount of o-anisole into the reaction kettle, and add glacial acetic acid under stirring. Add acetic anhydride dropwise at 10 to 12°C, finish the addition in about 2 hours, and let it stand for 2 hours to obtain the intermediate o-acetamidoanisole for industrial use. The ingredient ratio is anthranilate: glacial acetic acid: acetic anhydride = 1:0.9:1 (weight ratio). You can also mix anthranilate and acetic anhydride, reflux in a sand bath for 2 hours, let cool, obtain needle-like crystals, filter, wash away the acidity with water, and then recrystallize with water to obtain the finished product.
Purpose
1. Organic synthesis. Used as raw material for organic synthesis, used in the manufacture of pesticides, medicines, etc. Preparation of aniline yellow and other dyes.
