o-Methoxyacetoacetanilide
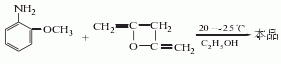
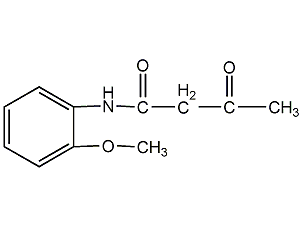
Structural formula
| Business number | 024E |
|---|---|
| Molecular formula | C11H13NO3 |
| Molecular weight | 207.23 |
| label |
2-Methoxy-N-acetoacetylaniline, N-(2-methoxyphenyl)-3-oxobutanamide; o-methoxyacetoacetaniline; acetoacetyl o-methoxyaniline, N-(2-Methoxyphenyl)-3-oxobutanamide, Acetoacetyl-o-acetanisidine, -Acetoacetamidoanisol, o-Acetoacetanisidide, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:92-15-9
MDL number:MFCD00008781
EINECS number:202-131-0
RTECS number:BZ5600000
BRN number:None
PubChem number:24891363
Physical property data
1. Properties: White powdery crystal.
2. Density (g/mL, 25/4℃): 1.1320
3. Relative vapor density (g/mL, air =1): Undetermined
4. Melting point (ºC): 86.6
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 163
9. Specific rotation (º): Undetermined
10 . Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12 . Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/water ) Log value of distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion Lower limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, chloroform and benzene, slightly soluble in ether.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 56.49
2. Molar volume (cm3/mol): 176.6
3. Isotonic specific volume (90.2K ): 452.3
4. Surface tension� (dyne/cm): 42.9
5. Polarizability (10-24cm3): 22.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 8
6. Topological molecule polar surface area 55.4
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 240
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
Stored in sealed and exposed light.
Packed in plastic-coated fiberglass bags, 30kg per bag. Place in a dry, ventilated place, away from sunlight. Handle with care when transporting. Protect from moisture and heat. Store and transport according to flammable and drug regulations.
Synthesis method
The following two process routes can be used. (1) Obtained from the reaction between ethyl acetoacetate and o-methoxyaniline. First, heat the ethanol solution of dry xylene, ethyl acetoacetate and triethanolamine to a slight boil, steam out part of the xylene solution (temperature is between 118-120°C), slowly heat it to 137-140°C, and collect the Cool the mixture of toluene and ethanol to 5-7°C, filter and crystallize, wash with a little xylene to obtain crude product, and then recrystallize with ethanol. (2) Reaction of o-methoxyaniline and diketene: add 500 parts of water to the reaction kettle. Add 0.02 part of triphenylphosphine and 0.1 part of N,N-dimethylaniline under vigorous stirring. Within 45 minutes, 90 parts of diketene (95.6% purity) and 1123 parts of o-methoxyaniline were added through two lines (inserted into the solution) under stirring (both were added at equimolar speeds). Cool sufficiently to keep the reaction temperature at 20°C. After the addition reaction is completed, stir for another 1 hour to obtain a slurry product. Cool it to 10°C, filter and dry to obtain 192 parts of white o-methoxyacetoacetanilide, with a yield of 93% and a purity of 99.5%. In industrial production, o-methoxyaniline and diketene can also be condensed in ethanol medium at 20-25°C.
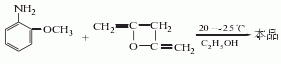
Purpose
1. Azo dye intermediates. Organic Synthesis.
