Oleylamine Oleylamine
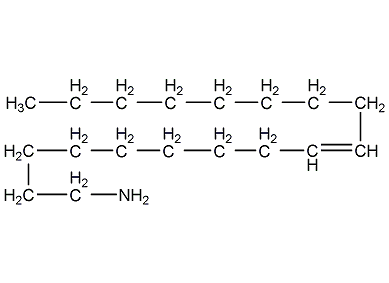
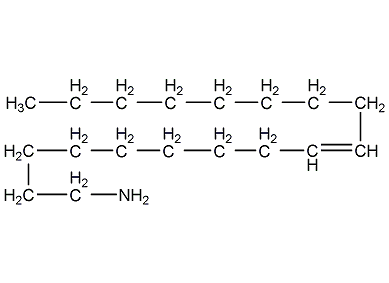
Structural formula
| Business number | 036W |
|---|---|
| Molecular formula | C18H37N |
| Molecular weight | 267.49 |
| label |
9-octadecylamine, Decacarbon-9-enamide, cis-1-Amino-9-octadecene, softener, waterproofing agent, Dispersant, Rust inhibitor, Chemical additives |
Numbering system
CAS number:112-90-3
MDL number:MFCD00066507
EINECS number:204-015-5
RTECS number:RG4130000
BRN number:1723960
PubChem number:24898049
Physical property data
1. Characteristics: White powder or flakes.
2. Density (g/mL,25℃):0.813
3. Relative vapor density (g/mL, Air =1): Undetermined
4. Melting point (ºC):18- 26
5. Boiling point (ºC,normal pressure):348-350
6. Boiling point (ºC,KPa):
7. Refractive index:1.4596
8. Flashpoint (ºC):
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,135ºC): 8
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Topological molecule polar surface area ( TPSA):26
7. Heavy atoms Quantity: 19
8. Surface charge :0
9. Complexity :175
10. Number of isotope atoms:0
11. Determine the number of atomic stereocenters:0
12. Uncertain number of atomic stereocenters:0
13. Determine the number of stereocenters of chemical bonds:1
14. Uncertain number of chemical bond stereocenters:0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
Place 100 kgOleic acid is put into the reaction kettle, heated and melted, and heated up under stirring. When the material reaches 180 ℃ or so, start ammonia flow. Ammonia gas enters the feed liquid from the bottom of the reactor through the distributor. Rapid stirring enhances the contact between the gas phase and the liquid phase. The by-product water and ammonia gas from the end melon enter the recovery device through the condenser. When the ammonia input amount is200 kg, detect whether there is water in the discharged gas phase. If there is no water in the gas, it is determined that the reaction has reached the end point. Stop nitrogen flow. Discharge while hot and cool to form. Use ethanol as solvent for recrystallization to obtain fine product.
Purpose
This product can be used as a softener and waterproofing agent for chemical fibers. It can also be used as dye, paint dispersant and metal rust inhibitor. Organic intermediates and textile auxiliaries.
mso-margin-top-alt: auto; mso-margin-bottom-alt: auto; mso-list: l0 level1 lfo1; tab-stops: list 18.0pt” align=left>13. Determine the number of stereocenters of chemical bonds:1
14. Uncertain number of chemical bond stereocenters:0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
Place 100 kgOleic acid is put into the reaction kettle, heated and melted, and heated up under stirring. When the material reaches 180 ℃ or so, start ammonia flow. Ammonia gas enters the feed liquid from the bottom of the reactor through the distributor. Rapid stirring enhances the contact between the gas phase and the liquid phase. The by-product water and ammonia gas from the end melon enter the recovery device through the condenser. When the ammonia input amount is200 kg, detect whether there is water in the discharged gas phase. If there is no water in the gas, it is determined that the reaction has reached the end point. Stop nitrogen flow. Discharge while hot and cool to form. Use ethanol as solvent for recrystallization to obtain fine product.
Purpose
This product can be used as a softener and waterproofing agent for chemical fibers. It can also be used as dye, paint dispersant and metal rust inhibitor. Organic intermediates and textile auxiliaries.
