oxalic acid
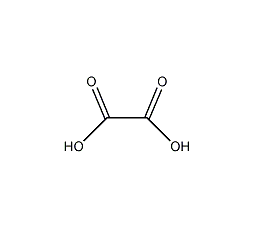

Structural formula
| Business number | 03WJ |
|---|---|
| Molecular formula | C2H2O4. |
| Molecular weight | 90.03 |
| label |
oxalic acid, Ethanedioic acid, Solvent for refining rare metals, dye reducing agent, tanning agent, acidity regulator |
Numbering system
CAS number:144-62-7
MDL number:MFCD00002573
EINECS number:205-634-3
RTECS number:RO2450000
BRN number:385686
PubChem number:24886864
Physical property data
1. Properties: colorless, odorless, hygroscopic substance, monoclinic flakes, prism crystals or white particles, easily weathered. Changes to dihydrate in air.
2. Melting point (ºC): 189.5 [α type (rhombus)]
3. Melting point (ºC): 182 [β type (monoclinic form)]
4. Relative density (g/mL, 17ºC): 1.900 (α type)
5. Relative density (g/mL, 17ºC): 1.895 (β type)
6. Refractive index (20ºC): 1.540
7. Heat of combustion (KJ/mol, 25ºC): -245.61
8. Standard heat of formation (KJ/mol, 25ºC) : -826.78
9. Heat of solution (KJ/mol, water): -9.58
10. Heat of sublimation (KJ/mol): 90.58
11 . Heat of decomposition (KJ/mol): 826.78
12. Thermal conductivity (W/(m·K),0ºC): 0.9
13. Solubility: easily soluble in Ethanol, soluble in water, slightly soluble in ether, insoluble in benzene and chloroform.
Toxicological data
1. Acute toxicity: rat oral LD50: 7500 mg/kg; mouse intraperitoneal LC50: 270 mg/kg;
2. Irritation data: skin – rabbit 500 mg/24 hours light Degree; Eyes – Rabbit 0.25 mg/24 hours Severe
3. It is corrosive and irritating to the skin and mucous membranes. Inhalation of steam and dust can cause poisoning. Swallowing can cause gastroenteritis, vomiting and diarrhea. and other symptoms. The lowest lethal dose for adults is 71 mg/kg.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies. This product is highly irritating and corrosive. Its dust or concentrated solution can cause serious damage to the skin, eyes or mucous membranes. It is highly toxic and corrosive. The lowest lethal dose of oxalic acid to humans is 71 mg/kg, and the lethal dose to adults is 15 to 30 g. If a person takes 5g of oxalic acid and oxalic acid orally, symptoms such as gastroenteritis, collapse, convulsions, shock and even death will occur. People who suffer from chronic poisoning by inhaling oxalic acid vapor will have symptoms such as extreme weakness, ulcers on the nasal mucosa, cough, body pain, vomiting, weight loss, and protein in the urine.
��Mainly used for the production of drugs such as antibiotics and borneol, as well as solvents for refining rare metals, dye reducing agents, tanning agents, etc. In addition, oxalic acid can also be used to synthesize various oxalate esters, oxalates, and oxalamides, among which diethyl oxalate, sodium oxalate, and calcium oxalate have the largest yields. Oxalic acid can also be used in the production of cobalt-molybdenum-aluminum catalysts, metal and marble cleaning, and textile bleaching. Acidity regulator used in the synthesis of urea-formaldehyde resin, melamine formaldehyde resin and other adhesives. It is used in the pharmaceutical industry to manufacture drugs such as oxytetracycline and chlortetracycline.
2. Mainly used as reducing agent and bleaching agent, mordant in printing and dyeing industry, also used to refine rare metals, synthesize various oxalate esters, oxalates and oxamides, etc.
3. Used as an acidity regulator for synthetic urea-formaldehyde resin, melamine formaldehyde resin and other adhesives. It can also be added to polyvinyl formal water-soluble adhesive to improve drying speed and bonding strength. It is an important raw material for organic synthesis and is used to produce various oxalates, oxalate esters, oxamides, hydroquinone, pentaerythritol, gallic acid and other products. The pharmaceutical industry is used to manufacture chlortetracycline, oxytetracycline, tetracycline, streptomycin, borneol, vitamin B12, phenobarbital and other drugs. In the printing and dyeing industry, it is used as color development aid and bleaching agent. The plastic industry is used to produce polyvinyl chloride, amino plastics, and urea-formaldehyde plastics. In the metallurgical industry, it is used to produce high-purity nickel, carbon rods and precipitated rare earth metals. It is also used as metal washing treatment agent, tanning agent, chelating agent, catalyst, anaerobic adhesive and polymerization inhibitor of acrylic quick-setting adhesive, machining rust remover, electroplating complexing agent, wood bleaching, marble cleaning and Oil refining, etc.
4. It can be used as an additive in shampoos in cosmetics; it is mainly used in the manufacturing of chemical industry products. It is used in the pharmaceutical industry to manufacture antibiotics and other drugs. It is widely used in the printing and dyeing industry and leather industry; it is used in oleochemistry. Refined preparations of terpineol, glycerin and stearate, etc.; in addition, they are used in aluminum product processing and cemented carbide production.
