p-Chloroacetophenone p-Chloroacetophenone
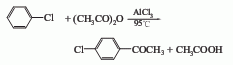

Structural formula
| Business number | 02GR |
|---|---|
| Molecular formula | C8H7ClO |
| Molecular weight | 154.59 |
| label |
p-Chloroacetylbenzene, 4′-Chloroacetophenone, 4-Chloroacetophenone, p-Chloroacetophenone, 1-(4-Chlorophenyl)-ethanon, 1-(4-Chlorophenyl)ethanone[qr], Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:99-91-2
MDL number:MFCD00000624
EINECS number:202-800-7
RTECS number:KM5600000
BRN number:386014
PubChem number:24853689
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25℃): 1.192
3. Relative density (20℃, 4℃): 1.1992
4. Melting point (ºC): 20
5. Boiling point (ºC, normal pressure): 234
6. Relative density (25℃, 4℃): 1.1885
7. Refractive index: 1.554
8. Flash point (ºC): 90
9. Refractive index at room temperature (n20): 1.5550
10. Refractive index at room temperature (n25): 1.5528
11. Vapor pressure (mmHg, 90 ºC): 8
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in organic solvents .
Toxicological data
1. Acute toxicity: Human inhalation TCL0: 1mg/m3/4H; Mouse oral LD50: 1207mg/kg Mouse inhalation LC50: 1752mg/m3/15M; Mouse peritoneal cavity LD50: 100mg/kg; 2. Other multiple doses Toxicity: mice inhaled TCLo: 87600μg/m3/15M/10D-I;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 41.17
2. Molar volume (cm3/mol): 132.9
3. Isotonic specific volume (90.2K ): 328.3
4. Surface tension (dyne/cm): 37.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 125
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants.
2.This product is toxic and strongly irritates the eyes, nose and throat. Protective glasses, protective clothing, and protective gloves should be worn. Combustion produces irritating, corrosive/toxic gases.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. Packed in iron drums, 200kg/drum.
Synthesis method
Obtained from the reaction of chlorobenzene and acetic anhydride: Heat and stir anhydrous aluminum trichloride, anhydrous carbon disulfide and dry chlorobenzene, and slowly drop in acetic anhydride while it is slightly boiling. After the addition, stir and reflux for 1 hour to recover carbon disulfide. When the reactants are cooled to room temperature and warm, slowly pour them into crushed ice containing hydrochloric acid while stirring. The layers should be clear. If they are not clear, add a little hydrochloric acid. Pour a small amount of benzene into this solution and extract, collect the oil layer, and extract the water layer again with benzene. Combine the extract and the oil layer, wash away the acidity with about 15% sodium hydroxide, then wash with water until neutral, and dry. After distillation under reduced pressure, collect the crude fraction, freeze it for 48 hours, separate the mother liquor, and melt the crystals to obtain the finished product. The yield is 83.1%.
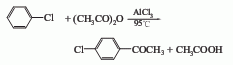
Purpose
Used as fluorescent whitening agent, pharmaceutical and intermediate raw material
