p-chlorobenzyl alcohol
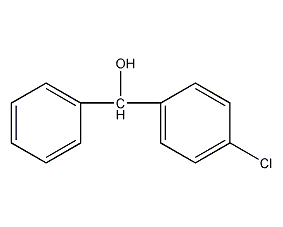

Structural formula
| Business number | 03B1 |
|---|---|
| Molecular formula | C13H11ClO |
| Molecular weight | 218.68 |
| label |
ClC6H4CH(C6H5)OH, Heterocyclic compounds |
Numbering system
CAS number:119-56-2
MDL number:MFCD00004491
EINECS number:204-333-4
RTECS number:None
BRN number:None
PubChem number:24847638
Physical property data
1. Character: Undetermined 2. Density (g/mL,25℃): Undetermined 3. Relative vapor density (g/mL,air=1 ): Undetermined 4. Melting point (ºC): 58-60 5. Boiling point (ºC,Normal pressure): Undetermined 6. Boiling point (ºC, 2.6mmHg):150-151 7. Refractive index: Undetermined 8. Flashpoint (ºC): Undetermined 9. Specific rotation (º): Undetermined 10. Autoignition point or ignition temperature (ºC): Undetermined 11. Vapor pressure (mmHg,ºC): Undetermined 12. Saturated vapor pressure (kPa, ºC): Undetermined 13. Heat of combustion (KJ/mol): Undetermined 14. Critical temperature (ºC): Undetermined 15. Critical pressure (KPa): Undetermined 16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined 17. Explosion upper limit (%,V/V): Undetermined 2
3、 Isotonic specific volume (90.2K) :169.5
4、 Surface Tension (dyne/cm):47.3
5、 Polarizability (10-24cm3):24.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 181
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
It is obtained by condensation and reduction of benzoyl chloride. 1.Condensation in a dry reaction pot, add chlorobenzene and anhydrous aluminum trichloride, stir and raise the temperature to 50℃ , add benzoyl chloride dropwise. After addition, react at 100-110℃ 5.5h, slowly put it into slightly acidic ice water, stir well and let it stand , separate the supernatant, filter the precipitate, wash until neutral, and spin dry. Get 4- chlorobenzophenone. The yield is 95%. 2.Reduction Add 4-chlorobenzophenone and liquid alkali into the reaction pot, stir and heat to 100℃, slowly add zinc powder, and control the temperature at 110-125℃. After the addition, the reaction time is 8h. Leave to stand, separate the oil layer, wash with hot water and a small amount of hydrochloric acid, then wash with hot water until pH is 6.5, and add benzene Stir to dissolve. Leave to stand, separate the benzene layer, steam the benzene, and the remaining liquid is the finished product.
Purpose
Used in the manufacture of the anti-allergic drug cetirizine.
AMILY: Arial; mso-font-kerning: 0pt”>10-24cm3): 24.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 181
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
It is obtained by condensation and reduction of benzoyl chloride. 1.Condensation in a dry reaction pot, add chlorobenzene and anhydrous aluminum trichloride, stir and raise the temperature to 50℃ , add benzoyl chloride dropwise. After addition, react at 100-110℃ 5.5h, slowly put it into slightly acidic ice water, stir well and let it stand , separate the supernatant, filter the precipitate, wash until neutral, and spin dry. Get 4- chlorobenzophenone. The yield is 95%. 2.Reduction Add 4-chlorobenzophenone and liquid alkali into the reaction pot, stir and heat to 100℃, slowly add zinc powder, and control the temperature at 110-125℃. After the addition, the reaction time is 8h. Leave to stand, separate the oil layer, wash with hot water and a small amount of hydrochloric acid, then wash with hot water until pH is 6.5, and add benzene Stir to dissolve. Leave to stand, separate the benzene layer, steam the benzene, and the remaining liquid is the finished product.
Purpose
Used in the manufacture of the anti-allergic drug cetirizine.
