p-Nitrophenetole 4-Nitrophenetole
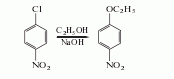
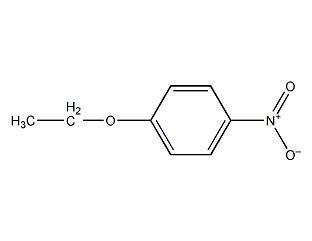
Structural formula
| Business number | 02HM |
|---|---|
| Molecular formula | C8H9NO3 |
| Molecular weight | 167.16 |
| label |
p-Nitrophenyl ether, 4-Nitrophenylethyl ether, 1-ethoxy-4-nitrobenzene, p-Nitroethoxybenzene, p-Ethoxynitrobenzene, P-Nitrophenetole, 4-Nitrophenetol, 4-Nitrophenetole, 1-Ethoxy-4-nitrobenzene, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:100-29-8
MDL number:MFCD00007330
EINECS number:202-837-9
RTECS number:DA0600000
BRN number:972473
PubChem ID:None
Physical property data
1. Character: light yellow columnar crystal [1]
2. Melting point (℃): 59~60[2]
3. Boiling point (℃): 283[3]
4. Relative density (water=1): 1.18[4]
5. Heat of combustion (kJ/mol): -4205.1[5]
6. Octanol/water partition coefficient: 2.53 [6]
7. Solubility: insoluble in water, soluble in ethanol, soluble in hot petroleum ether, easily soluble in hot ethanol and ether, miscible in acetone and benzene. [7]
Toxicological data
1. Acute toxicity: rat oral LD50: 3300mg/kg; rat skin contact LD50: >16mg/kg; rat peritoneal cavity LD50: 2100mg/kg; rabbit skin contact LD50: >7940mg/kg;
2. Mutagenicity: Mutant microorganism test: bacteria-Salmonella typhimurium, 250μg/plate; DNA repair test: Bacillus subtilis, 5mg/disc.
3. Acute toxicity [8]
LD50: 3300mg/kg (rat oral); >16g/ kg (rat transdermal); >7940mg/kg (rabbit transdermal)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 44.10
2. Molar volume (cm3/mol): 141.7
3. Isotonic specific volume (90.2K ): 359.2
4. Surface tension (dyne/cm): 41.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 17.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 55
7. Number of heavy atoms: 12
8. Surface charge: 0
9. ComplexDegree: 147
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain atomic configuration Number of centers: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalent Number of key units: 1
Properties and stability
1. Stability[9] Stable
2. Incompatible substances[10] Strong oxidants, strong reducing agents, strong acids, strong bases
3. Conditions to avoid contact[11] Heat
4. Polymerization hazard[12] No polymerization
5. Decomposition products[13] Nitrogen oxides
Storage method
1. Storage precautions[14] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packaging adopts small opening steel drums, screw-top glass bottles, iron-top pressure-top glass bottles, plastic bottles or wooden crates outside metal drums (cans). Store in a cool, ventilated warehouse. Keep away from fire and heat sources, prevent direct sunlight, and keep the container tightly sealed. Load and unload with care when transporting.
Synthesis method
Prepared by etherification of p-nitrochlorobenzene and ethanol in the presence of sodium hydroxide. Add p-nitrochlorobenzene and ethanol into the reaction kettle, raise the temperature to 82°C, add ethanol sodium hydroxide solution dropwise, and react at 85-88°C for 3 hours. The alkalinity of the reaction solution drops below 0.9%, the temperature is lowered to 75°C, and the pH value is adjusted to 6.7-7 with concentrated hydrochloric acid. After standing for stratification, take the oil layer, wash it with heated water to remove the sodium nitrophenolate, carry out vacuum distillation of the oil layer, and take the 214-218℃ (2.66-5.32kpa) fraction as this product.
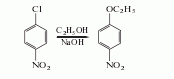
Purpose
1. Used as an intermediate for drugs and dyes. Used in medicine to synthesize phenacetin, etc.
2. Used as dye intermediate. [15]
