p-nitrophenylacetic acid p-nitrophenylacetic acid
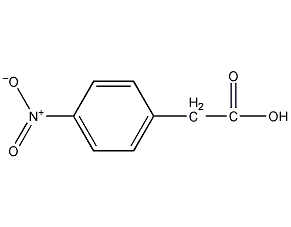

Structural formula
| Business number | 02PM |
|---|---|
| Molecular formula | C8H7NO4 |
| Molecular weight | 181.15 |
| label |
p-Nitrophenylacetic acid, 4-Nitrophenylacetic acid, p-Niphenylacetic acid, P-Nitrophenylacetic acid, Nitrophenylacetic acid(4-), (p-Nitophenyl)aceticacid, (P-Nitrophenyl)-aceticaci, 2-(P-Nitrophenyl)aceticacid, 4-Nitro-benzeneaceticaci, 4-Nitrophenylessigsαure |
Numbering system
CAS number:104-03-0
MDL number:MFCD00007383
EINECS number:203-168-5
RTECS number:AJ1130010
BRN number:1911801
PubChem ID:None
Physical property data
1. Properties: light yellow needle-like crystals
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, Air=1): Undetermined
4. Melting point (ºC): 153-156
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 3mmHg): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9 . Specific rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined Determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical Temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
p>
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Dissolution Properties: Soluble in hot water, ethanol and benzene, slightly soluble in cold water.
Toxicological data
1. Acute toxicity: rat oral LD: >500mg/kg; mouse peritoneal cavity LD50: 830mg/kg; 2. Mutagenicity: DNA repair test: Bacillus subtilis, 500μg/disc;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 43.91
2. Molar volume (cm3/mol): 128.7
3. etc.�Specific volume (90.2K): 361.1
4. Surface tension (dyne/cm) 61.9
5. Dielectric constant:
6. Dipole Distance (10-24cm3):
7. Polarizability: 17.40
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 80.4
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 203
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from the hydrolysis of p-nitrophenyl acetonitrile. Mix p-nitrobenzene acetonitrile, concentrated sulfuric acid and water, and heat to reflux for 15 minutes. Then pour the reactant into ice water, precipitate the crystals, filter, wash the filter cake with ice water, and then recrystallize with water to obtain p-nitrophenyl acetic acid. The yield is over 92%.
2. Preparation method:
In a reaction bottle equipped with a stirrer and a reflux condenser, add 150 mL of water, and slowly add 150 mL of concentrated sulfuric acid. Then, 50 g (0.3 mol) of p-nitrobenzene acetonitrile (2) was added, and the reaction was heated and refluxed for 0.5 h with stirring. After the reaction is completed, add an equal volume of water, cool and filter the precipitated liquid. Recrystallize with about 1000 mL hot water, decolorize with activated carbon, filter while hot, and cool to crystallize. Filter with suction, wash with water and dry to obtain 53g of light yellow needle crystal p-nitrophenyl acetic acid (1), mp 151~153°C, yield 95%. [1]
Purpose
Used in organic synthesis.
