p-t-Pentylphenol p-t-Pentylphenol
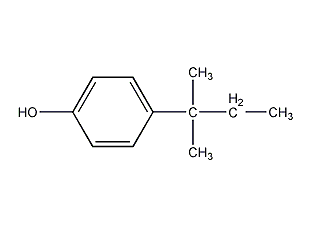

Structural formula
| Business number | 01R3 |
|---|---|
| Molecular formula | C11H16O |
| Molecular weight | 164.24 |
| label |
4-(1,1-dimethylpropyl)phenol, 4-tert-amylphenol, 4-(1,1-Dimethylpropyl)-phenol, p-tert-Pentylphenol |
Numbering system
CAS number:80-46-6
MDL number:MFCD00002369
EINECS number:201-280-9
RTECS number:SM6825000
BRN number:1908224
PubChem number:24849381
Physical property data
1. Properties: White needle-like crystals.
2. Density (g/mL, 20/4℃): 0.962
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 94.5
5. Boiling point (ºC, normal pressure): 262.5
6. Gas phase standard heat of combustion (enthalpy) (kJ·mol-1): -6327.47
7. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -233.6
8. Flash point (ºC, open cup): 111
9. Specific rotation (º): Undetermined
10. Solid phase standard heat of combustion (enthalpy) ( kJ·mol-1): -6310.53
11. The solid state standard claims heat (enthalpy) (kJ·mol-1): -305.7
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature ( ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether, benzene and chloroform, almost insoluble in water.
Toxicological data
Rat caliber LD50: 1830mg/kg;
Rabbit skin LD50: 2 mg/kg;
2. Neurotoxicity
Rabbit skin test: 100mg/24H; Rabbit eye test: 1%REACTION SEVERITY
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 51.15
2. Molar volume (cm3/mol): 171.0
3. Isotonic specific volume (90.2K ): 410.1
4. Surface tension (dyne/cm): 33.0
5. Polarizability (10-24cm3):20.27
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity :132
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units :1
Properties and stability
1. Exist in mainstream smoke.
2. It is corrosive and pollutes the environment. Oral administration to rats is LD503.08g/kg.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
1. Prepared by the condensation of tert-amyl alcohol and phenol.
Purpose
Organic synthesis. Preparation of oil-soluble resins, fumigants, synthesis of organic mercury fungicides, pesticides and rubber intermediates.
