phenyl benzoate
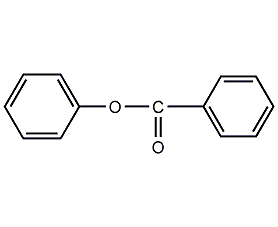

Structural formula
| Business number | 0270 |
|---|---|
| Molecular formula | C13H10O2 |
| Molecular weight | 198.22 |
| label |
phenyl benzoate, phenyl benzoate, Benzoic acid phenyl ester, C6H5CO2C6H5 |
Numbering system
CAS number:93-99-2
MDL number:MFCD00003072
EINECS number:202-293-2
RTECS number:DH6299500
BRN number:1566346
PubChem number:24847623
Physical property data
1. Properties: colorless prismatic crystals. It smells like grass oil.
2. Relative density (20℃, 4℃): 1.235
3. Relative density (25℃, 4℃): 1.041131
4. Melting point (ºC): 71
5. Boiling point (ºC, normal pressure): 314
6. Boiling point (ºC, 2.0kpa): 314℃
7. Refractive index at room temperature (n20): 1.550280
8. Refractive index at room temperature (n 25): 1.5403102
9. Specific rotation (º): Undetermined
10. Gas phase standard heat of combustion (enthalpy) (kJ·mol-1): -6402.2
11. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -142.6
12. The standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -6303.2
13. The standard claim heat of the crystal phase ( Enthalpy) (kJ·mol-1): -241.6
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa) : Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
p>
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot ethanol, slightly soluble in cold ethanol and ether, insoluble in water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 57.89
2. Molar volume (cm3/mol): 172.8
3. Isotonic specific volume (90.2K ): 444.6
4. Surface tension (dyne/cm): 43.8
5. Polarizability (10-24cm3): 22.95
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7.Number of heavy atoms���:15
8. Surface charge: 0
9. Complexity: 201
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed.
Synthesis method
Obtained from the reaction of benzoyl chloride and phenol. Dissolve phenol in pyridine, add benzoyl chloride dropwise, control the temperature not to exceed 50°C, complete the addition, and react at 120°C for 30 minutes. After cooling, add water to precipitate crystals, filter, wash and dry to obtain phenyl benzoate. The yield is 93%. The above-mentioned acylation reaction can also be carried out by reacting phenol sodium salt solution and benzoyl chloride at room temperature for 0.5 hours. After filtering and washing with water, phenyl benzoate can be obtained. For refinement, ethanol recrystallization can be used, and the yield is 75-80%.
Purpose
This product is heated to 130°C in the presence of aluminum trichloride and rearranged to form 4-hydroxybenzophenone, which is used as an intermediate in organic synthesis and in the production of pharmaceuticals and other products.
