Pimelic acid Pimelic acid
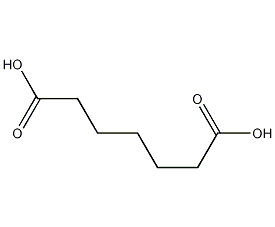

Structural formula
| Business number | 032V |
|---|---|
| Molecular formula | C7H12O4 |
| Molecular weight | 160.17 |
| label |
Syzygous acid, 1,5-Pentanedicarboxylic acid, 1,7-Pimelic acid, 1,7-Heptanedioic acid, 1,5-pentanedicarboxylic acid, Heptanedioic acid, aliphatic compounds, acidic solvent |
Numbering system
CAS number:111-16-0
MDL number:MFCD00004425
EINECS number:203-840-8
RTECS number:TK3677000
BRN number:1210024
PubChem number:24887550
Physical property data
1. Properties: colorless monoclinic columnar crystal
2. Density (g/mL, 20℃): 1.329
3. Relative density (20℃, 4℃ ): 1.32915
4. Melting point (ºC): 105.7~105.8
5. Boiling point (ºC, normal pressure): 272100
6. Boiling point (ºC, 13.3kPa): 212 (1333pa)
7. Crystal phase standard combustion heat (enthalpy) (kJ·mol -1): -3460.17
8. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -1009.39
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): 568.85
15. Critical pressure (MPa): 3.28
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility : Easily soluble in alcohol and ether, almost insoluble in cold benzene.
20. Flash point (ºC): 181.9
Toxicological data
1. Acute toxicity: Rat oral LD5O: 7gm/kg
Mouse oral LD5O: 4800mg/kg
Ecological data
Slightly harmful to water.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 37.60
2. Molar volume (cm3/mol): 133.3
3. Isotonic specific volume (90.2K): 354.2
4. Surface tension (dyne/cm): 49.8
5.Polarizability (10-24cm3): 14.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 125
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides.
2. Found in flue-cured tobacco leaves, burley tobacco leaves, and oriental tobacco leaves.
3. Can be sublimated.
Storage method
Stored sealed in a cool, dry place. Ensure that the workspace has good ventilation facilities and is stored away from sources of fire and oxidants.
Ecology: Slightly harmful to water.
Synthesis method
1. Put metallic sodium into isoamyl alcohol at 100°C, continue heating to cause vigorous reflux, and add dropwise a solution of salicylic acid dissolved in isoamyl alcohol. After the reflux reaction is completed, cool to 100°C, add hot water while stirring, let it stand, separate the water layer for steam distillation, remove all the isoamyl alcohol, and collect the distillate until a certain amount is reached. Cool the contents in the distillation bottle, add concentrated hydrochloric acid, and then perform steam distillation to remove salicylic acid. The residue is then concentrated until sodium chloride is precipitated, and a mixture of pimelic acid and sodium chloride is precipitated by cooling. The mixture was extracted with benzene, and pimelic acid was concentrated and crystallized from the extract. The yield is 43-50%.
2. Tobacco: OR, 44; FC, 54; BU, 26.
3. Obtain monoclinic prisms from benzene.
4. Preparation method:
In a reaction bottle equipped with a stirrer and a reflux condenser, add 18g (0.148mol) of pimelonitrile (2), 50% (mass) 250g of sulfuric acid, heated to reflux with stirring for 9 hours. A solid precipitates after cooling. filter. The filtrate was saturated with ammonium sulfate, extracted with ether three times, 50 mL each time, and the ether layers were combined. Dissolve the filtered solid in the diethyl ether layer, dry it over anhydrous sodium sulfate, evaporate the solvent, and recrystallize the remaining solid with benzene containing 5% diethyl ether to obtain 22g of pure pimelic acid (1), mp105~106 ℃, yield 93%. [1]
Purpose
For biochemical research.
