Piperidolate Hydrochloride Piperidolate Hydrochloride
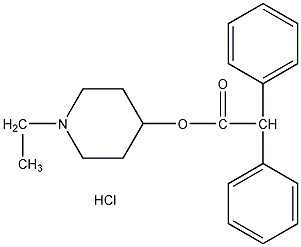

Structural formula
| Business number | 03M6 |
|---|---|
| Molecular formula | C21H26ClNO2 |
| Molecular weight | 359.90 |
| label |
Diheperdone, aromatic compounds |
Numbering system
CAS number:129-77-1
MDL number:MFCD00057377
EINECS number:204-964-5
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Characteristics: colorless flammable liquid.
2. Density (g/mL ,25/4℃): 0.7681
3. Refractive index (n D20): 1.3900
4. Flashpoint (℃):-15
5. Melting point (℃):-112
6. Boiling point (ºC):83
7. Solubility:Easily soluble in ethanol and ether, slightly soluble in water.
8. Stability:Chemically active, easy to undergo addition and polymerization reactions, easily hydrolyzed by acids into acetaldehyde and Isobutanol.
Toxicological data
1, acute toxicity: rat intravenously LD5O: 19mg/kg Mice orally LD5O: 1040mg/kg Mouse transvenous LD5O: 26mg/kg Dog Meridian VeinLD5O:35mg/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 367
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
None yet
Synthesis method
Introduction to production methods
Produced by the base-catalyzed addition of acetylene and isobutanol.
Purpose
Usage
Used as polymer monomer and organic synthesis intermediate.
AMILY: Times New Roman; mso-list: Ignore”>8. Stability:Reactive chemical properties, prone to addition and polymerization reactions, and easily hydrolyzed by acids into acetaldehyde and isobutanol.
Toxicological data
1, acute toxicity: rat intravenously LD5O: 19mg/kg Mice orally LD5O: 1040mg/kg Mouse transvenous LD5O: 26mg/kg Dog Meridian VeinLD5O:35mg/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 367
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
None yet
Synthesis method
Introduction to production methods
Produced by the base-catalyzed addition of acetylene and isobutanol.
Purpose
Usage
Used as polymer monomer and organic synthesis intermediate.
