Potassium ethyl xanthate Potassium ethyl xanthate
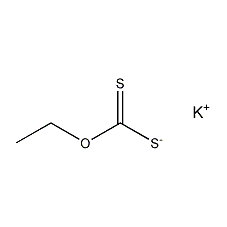

Structural formula
| Business number | 03TF |
|---|---|
| Molecular formula | C3H5KOS2 |
| Molecular weight | 160.30 |
| label |
Potassium Ethyl Disulfide Carbonate, Pesticide emulsifier 600, Potassium xanthate, Potassium B xanthogenate, Potassium O-ethyldithiocarbonate, (o-Ethyldithiocarbonato)potassium, aliphatic compounds |
Numbering system
CAS number:140-89-6
MDL number:MFCD00004931
EINECS number:205-439-3
RTECS number:205-439-3
BRN number:3596974
PubChem number:24881784
Physical property data
1. Physical property data:
1.Properties: white to light yellow crystals or crystalline powder
2.Density (g/mL,25/4℃): 1.558
3 Melting point (℃): 210 ºC (decomposition)
4.Flash point (ºC): 96
5.Flash point or ignition temperature (ºC): 205
6. DissolveProperty: Easily soluble in water, soluble in ethanol
Toxicological data
2. Poison Physics data:
1, acute toxicity: rat oral LD50: 1700 mg/kg;
Mouse oralLD50 :308 mg/kg;
span> Mouse abdominal cavityLD50: >500 mg/kg;
Mouse subcutaneousLD50:400 mg/kg;
Mouse veinLD50: 199 mg/kg;
Rat OralLD50 : 910 mg/kg/26W-I.
Ecological data
3. Ecology Data:
1. Other harmful effects: This substance may be harmful to the environment, and special treatment should be given to water bodies. Notice.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 42.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 56.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure.
Storage method
Keep sealed and dry, away from oxidants.
Synthesis method
Add absolute ethanol and caustic potassium into the reaction pot and heat until fully dissolved. Then cool to20℃, add carbon disulfide, discharge while hot, cool naturally, precipitate crystals, filter, and vacuum dry to obtain potassium xanthate.
Purpose
1.An intermediate of thiamphenicol. Chemical analysis reagents.
2.Organophosphorus and organochlorine pesticides.
3.acts as a collector in mineral processing.
ly: Arial; mso-bidi-font-family: Arial”>Keep sealed and dry, away from oxidants.
Synthesis method
Add absolute ethanol and caustic potassium into the reaction pot and heat until fully dissolved. Then cool to20℃, add carbon disulfide, discharge while hot, cool naturally, precipitate crystals, filter, and vacuum dry to obtain potassium xanthate.
Purpose
1.An intermediate of thiamphenicol. Chemical analysis reagents.
2.Organophosphorus and organochlorine pesticides.
3.acts as a collector in mineral processing.
