Potassium oleate
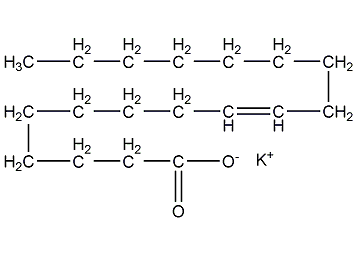

Structural formula
| Business number | 03VW |
|---|---|
| Molecular formula | C18H35KO2 |
| Molecular weight | 322.55 |
| label |
Potassium octadecenoate, (Z)-9-Octadenoic acid potassium salt, Potassium octadecenoate, potassium soap, soft soap, Potassium stearate, Potassiumcis-9-octadecenoic acid, Potassium stearate, Detergents, emulsifiers |
Numbering system
CAS number:143-18-0
MDL number:MFCD00064243
EINECS number:205-590-5
RTECS number:RK1150000
BRN number:4167152
PubChem number:24857521
Physical property data
1. Physical property data:
1. Flash point (ºC): 140ºC
2. Properties: light yellow or light brown solid
3. Solubility: soluble in water and ethanol, slightly soluble in cold water.
Toxicological data
2. Toxicological data:
1. Acute toxicity: Rat oral LD50: >5 mg/kg;
Rat dermal LD50: >2 mg/kg kg;
Mouse oral LC50: >5 mg/kg.
2. Inhalation toxicity: rat LD50: 5610mg/kg
Ecological data
3. Ecological data:
Usually it is not harmful to water. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
None
Compute chemical data
IV. Calculated chemical data:
1. Number of hydrogen bond donors: 0
2. Number of hydrogen bond acceptors: 2
3. Number of rotatable chemical bonds: 15
4. Topological molecular polar surface area (TPSA): 40.1
5. Number of heavy atoms: 21
6. Surface charge : 0
7. Complexity: 239
8. Number of isotope atoms: 0
9. Determine the number of atomic stereocenters: 0
10. The number of uncertain atomic stereocenters: 0
11. The number of determined chemical bond stereocenters: 1
12. The number of uncertain chemical bond stereocenters: 0
13. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: strong oxidizing agents
Storage method
Keep sealed.
Synthesis method
1. Mainly usedChemical method. It is obtained by saponifying butter, mutton fat and other fats with potassium hydroxide, salting out and separating glycerin.
Purpose
1. Used as emulsifier and cleaning agent. Used in cream and shampoo cosmetics. It has high emulsifying efficiency , but it easily forms calcium soap when exposed to hard water. It can also be used as fiber softener.
2. It is a potassium catalyst that is widely used in polyisocyanate foam reactions.
