Propargyl chloride
Structural formula
| Business number | 06R8 |
|---|---|
| Molecular formula | C3H3Cl |
| Molecular weight | 74.51 |
| label |
3-Chloro-1-propyne, Aliphatic halogenated derivatives |
Numbering system
CAS number:624-65-7
MDL number:MFCD00000980
EINECS number:210-856-9
RTECS number:None
BRN number:506005
PubChem number:24848620
Physical property data
1. Properties: Transparent brown liquid
2. Density (g/mL, 25/4℃): 1.0239
3. Relative density (20℃, 4℃) :1.0297
4. Melting point (ºC): -77
5. Boiling point (ºC, normal pressure): 57.5
6. Refractive index at room temperature (n 20): 1.4336
7. Refractive index: 1.458
8. Flash point (ºC): 32.2-35
9 . Non-temperature refractive index (n 25 ): 1.4317
10. Gas Standard Entropy (J. MOL -1 · K -1 <): 302.67
11. Gas phase standard hot melt (J·mol-1·K-1): 72.51
12. Liquid phase standard hot melt (J·mol-1·K-1): 108.8
13. Heat of combustion (KJ /mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water Log value of (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V /V): Undetermined
19. Solubility: Insoluble in water.
Toxicological data
None yet
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 18.62
2. Molar volume (cm3/mol): 73.2
3. Isotonic specific volume (90.2K ): 168.9
4. Surface tension (dyne/cm): 28.3
5. Polarizability (10-24cm3): 7.38
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 38.5
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Tear-inducing. Irritation to skin. Toxicity has not been reported. Wear protective equipment during operation to avoid direct contact. After touching the skin, rinse with plenty of water. The workplace should be well ventilated and equipment should be sealed.
2. In the presence of copper halide, propynyl halide can undergo partial isomerization reaction to obtain a mixture of propynyl halide and halogenated allene.
Storage method
Store in a dark place, protected from fire and heat. Store and transport according to dangerous goods regulations.
Synthesis method
1. Obtained from the reaction of propargyl alcohol and phosphorus trichloride. First add kerosene and phosphorus trichloride into a dry reaction tank, add dropwise the mixture of propargyl alcohol and pyridine below 20°C. After the addition is completed, raise the temperature to reflux. After 4 hours of reaction, add ice water and separate the water layer. Add sodium carbonate water to the oil layer and mix it to pH=5-6, separate the water layer, and then wash, dry, and distill under normal pressure to collect the 52-60°C fraction to obtain the finished product. Raw material consumption quota: propargyl alcohol (95%) 1360kg/t, phosphorus trichloride (96%) 1350kg/t.
[Preparation method] It is obtained by reacting propargyl alcohol with
phosphorus trichloride in the presence of pyridine. 
2. Preparation method:
![]()
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and dropping funnel, add kerosene 1.2 kg, 1kg of phosphorus trichloride, cool to below 15°C, slowly add a mixture of 1kg of propargyl alcohol (2) and 0.3kg of pyridine dropwise while stirring. After the addition is completed, the reaction is refluxed for 4 hours. Cool to 20°C, pour into ice water, and stir the ingredients. Let stand and separate the water layer. The oil layer is adjusted to pH 5-6 with 5% sodium carbonate aqueous solution, and the water layer is separated. The oil layer was washed twice with water, dried over calcium chloride, filtered, and distilled. The fractions at 50-60°C were collected to obtain propargyl chloride (1) with a yield of 60%. [1]
Purpose
1. Used as an intermediate in organic synthesis. Used in the manufacture of medicine Youjiangning, soil fumigant, etc. It is also a modifier for engineering plastics. Its trisodium salt is an excellent heat stabilizer for polyvinyl chloride. Its esters are also important additives for polymers.
2. Organic synthesis intermediates, such as used in the preparation of medicine Youjiangning, etc., can also be used to prepare soil fumigants.
3. Propargyl chloride is a three-carbon alkylating reagent, and it is also the equivalent of acetone carbene ion and a ring-enhancing reagent. When converted into a metal-organic reagent, it is an alkynyl or propyl group. Diene nucleophile.
As an electrophile, propargyl can be used to realize many substrates such as Grignard reagents, carbanions, enols, carbonyl compounds, alcohols, phenols, carboxylates, amines, amides, and sulfonamides , Alkynylation reactions of sulfones, thiols, sulfamides and alkyl phosphates[1]. Since this type of reaction is carried out under alkaline conditions, the isomerization reaction of propargyl chloride will inevitably occur to obtain chlorinated allene compounds. The degree of isomerization reaction depends on the reaction time, temperature and Type of base used.
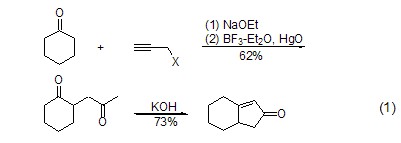
Propargyl halide can also be used in ring-enlarging reactions. For example, after the alkylation reaction between propargyl bromide and the substrate, mercury oxide is added to obtain an acetone-like alkylation product. When the alkylation reaction occurs at the ortho position of the carbonyl group, further intramolecular cyclization reaction can occur to obtain the cyclopentenone compound (formula 1)[2].
Due to the alkylation reaction The compound contains an alkynyl group, so it can further undergo intramolecular rearrangement reactions, such as the reaction in which the generated alkynyl vinyl ether undergoes [1,5]-hydrogen migration and rearranges into a ring-closed benzofuran (Formula 2)[3].
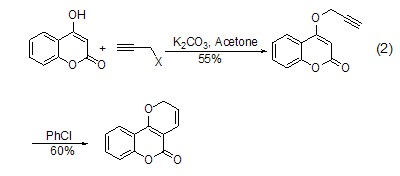
Propargyl chloride can act as a pro- The diene group undergoes an intramolecular Diels-Alder reaction with furan, styrene and triazabenzene[4]. Propargyl bromide can also participate in [3+2] cycloaddition reactions as a 1,3-dipolephile.
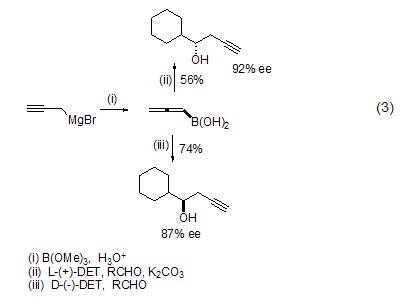
As a halide, propargyl bromide can also be converted into various organometallic reagents, and then undergo chiral addition reactions with aldehydes or ketones in the presence of diethyl tartrate (Formula 3)[5].

