Propionic Anhydride Propionic Anhydride
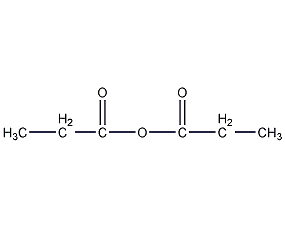

Structural formula
| Business number | 03GW |
|---|---|
| Molecular formula | C6H10O3 |
| Molecular weight | 130.14 |
| label |
Oleic anhydride, Propanoic anhydride, esterifying agent, dehydrating agent, Acid anhydride solvent |
Numbering system
CAS number:123-62-6
MDL number:MFCD00009303
EINECS number:204-638-2
RTECS number:UF9100000
BRN number:507066
PubChem ID:None
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -45[2]
3. Boiling point (℃): 167[3]
4. Relative density (water = 1): 1.02[4]
5. Relative vapor Density (air=1): 4.49[5]
6. Saturated vapor pressure (kPa): 0.13 (20.6℃)[6]
7. Heat of combustion (kJ/mol): -3120.8[7]
8. Critical temperature (℃): 342.7[8]
9. Critical pressure (MPa): 3.34[9]
10. Octanol/water partition coefficient: 0.400 [10]
11. Flash point (℃): 73.9 (OC); 63 (CC) [11]
12. Ignition temperature (℃): 285[12]
13. Explosion limit (%): 11.9[13]
14. Lower explosion limit (%): 1.48[14]
15. Solubility: soluble in ethanol, ether, chloroform, and alkali. [15]
16. Refractive index (n20ºC): 1.4038
17. Viscosity (mPa·s, 20ºC): 1.144
18. Viscosity (mPa·s, 30ºC): 1.061
19. Heat of evaporation (KJ/mol, b.p.): 41.74
20. Heat of generation (KJ/mol) : -555.59
21. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.80
22. Thermal conductivity (W/(m·K)) : 0.1244
23. Relative density (20℃, 4℃): 1.0110
24. Relative density (25℃, 4℃): 0.979150
25. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3163.69
26. Gas phase standard claimed heat (enthalpy) ( kJ·mol-1): -626.51
27. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1): -3111.14
28. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -679.06
29. Liquid phase standard hot melt (J ·mol-1·K-1): 233.7
Toxicological data
1. Acute toxicity[16]
LD50: 2360mg/kg (rat oral); 10ml/kg (rabbit oral) Skin)
2. Irritation [17]
Rabbit transdermal: 510mg, moderate irritation (open Stimulation test)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 17d (theoretical).
In an environment with a pH of 7 or 8,The solution half-lives are 10min and 1min respectively.
4. Other harmful effects [19] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 31.65
2. Molar volume (cm3/mol): 128.1
3. Isotonic specific volume (90.2K ): 300.6
4. Surface tension (dyne/cm): 30.2
5. Polarizability (10-24cm3): 12.54
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 104
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: When exposed to water and ethanol, it decomposes into propionic acid and propionate ester respectively.
2. Stability[20] Stable
3. Incompatible substances[21] Water, strong oxidants, strong alkali
4. Conditions to avoid contact[22] Humid air
5. Hazards of aggregation[23] No aggregation
Storage method
Storage Precautions[24] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, etc. and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is obtained by heating and dehydrating propionic acid at 235℃ and 8×105Pa. It can also be done by heating sodium propionate and propionyl chloride together to reflux for 4 hours, and then through distillation and fractionation, the 165-169°C fraction is collected as propionic anhydride.
Refining method: Shake with phosphorus pentoxide for a few minutes and then distill.
Purpose
1. Propionic anhydride is used as a propionylating agent in the manufacture of medicines, fragrances and special esters. It is used in the pharmaceutical industry to produce keratosamycin propionate (antibiotics) and testosterone propionate (androgen deficiency). ), oxymethasone propionate (anticancer drug), clodexamethasone dipropionate (adrenocortical hormones) and betamethasone dipropionate (adrenocortical hormones), etc. It is also used as a dehydrating agent in nitration and sulfonation reactions in organic synthesis, and in the manufacture of alkyd resins and dyes.
2. Used as esterification agent, dehydrator and in the manufacture of dyes, medicines and perfumes. [25]
