Salicylanilide


Structural formula
| Business number | 01XL |
|---|---|
| Molecular formula | C13H11NO2 |
| Molecular weight | 213.23 |
| label |
2-Hydroxy-N-phenylbenzylamine, N-phenyl salicylanilide, N-phenyl-2-hydroxybenzylamine, Ansadol,Hyanilid,Salifebrin,Salinidol,Shirlan, Fungicide |
Numbering system
CAS number:87-17-2
MDL number:MFCD00002212
EINECS number:201-727-8
RTECS number:VN7850000
BRN number:1108135
PubChem number:24848034
Physical property data
1. Properties: White leaf-shaped crystals. Odorless.
2. Melting point (ºC): 135.8~136.2
3. Solubility: Slightly soluble in water, easily soluble in alcohol, ether, benzene and chloroform
Toxicological data
Mouse caliber LD50: 2400mg/kg; Mouse abdominal cavity LD50: >500mg/kg
Ecological data
Has certain harm to water bodies.
Molecular structure data
1. Molar refractive index: 62.71
2. Molar volume (cm3/mol): 166.7
3. Isotonic specific volume (90.2K): 461.9
4. Surface tension (dyne/cm): 58.9
5. Polarizability (10-24cm3): 24.86
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 7
6. Topological molecule polar surface area 49.3
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 236
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
White leaf-like crystals. Easily soluble in alcohol, ether, benzene and chloroform, slightly soluble in water. Stable in the air, the color becomes darker when exposed to light. It can kill or inhibit general molds and bacteria. Its ability to inhibit molds is slightly weaker than carbendazim, but it can kill Mucor, etc. that grow under conditions of high relative humidity. Low toxicity to humans and animals.
Storage method
This product should be kept sealed, dry and protected from light.
Synthesis method
(1) Use salicylic acid and aniline as raw materials 1. Use toluene or carbon tetrachloride as the solvent for salicylic acid and aniline, and gradually add trichlorophosphine (salicylic acid: aniline: trichlorophosphine = 3:3:1, molar ratio). After the addition is completed, the temperature is raised and the boiling reaction is carried out until the hydrogen chloride gas no longer escapes. Stop reacting. Steam off the solventRecycling and recycling, the product is washed with cold water, filtered and dried to obtain the finished product rate. 2. Add 56g of newly distilled aniline into the three-necked bottle. Heat to 100-120°C with stirring to melt all the salicylic acid, then cool to 90-100°C, and add 20g of phosphorus trichloride dropwise for reaction. The hydrogen chloride generated by the reaction is introduced into the absorber and absorbed with water or alkali solution. After the dropwise addition is completed, raise the temperature to 140-150°C with stirring, and continue the reaction for 3 hours. Then put the reactant into cold water, and white crystals will precipitate. After filtering and drying, the finished product is obtained. 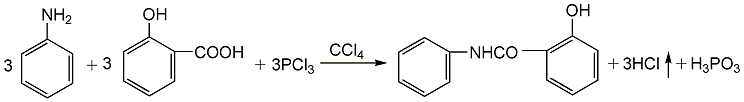 (2) Using acetylsalicylic acid and aniline as raw materials 1. Mix 1.8g (0.01mol) Add acetylsalicylic acid and 1.5ml (0.02mol) thionyl chloride into a 50ml reaction bottle and reflux until no bubbles are generated. Evaporate excess thionyl chloride to obtain a light yellow 2-acetoxybenzoyl chloride liquid. Add 5 ml of calcium chloride-dried acetone and set aside. 2. Add 1.4ml (0.01mol) triethylamine, 0.9g (0.01mol) aniline and 20ml dried acetone into a 1000ml reaction bottle, stir evenly, then slowly add 2-acetoxybenzene dropwise while cooling in an ice water bath. After the formyl solution is added dropwise, stir at room temperature for several hours. Evaporate the acetone under reduced pressure, add an appropriate amount of water, stir for a few minutes, let it stand, filter with suction, and wash with water. Hydrolyze the solid with 2 mol/L sodium hydroxide solution at room temperature for 4 hours, filter out the insoluble matter, and adjust the pH value of the filtrate to 4 with 3 mol/L hydrochloric acid. Filter with suction, wash with water, and recrystallize the solid with ethanol-water to obtain 2.2 g of white granular solid, with a yield of 80.6%. Aniline salicylate can also be produced by reacting methyl salicylate, phenyl salicylate and newly distilled aniline.
(2) Using acetylsalicylic acid and aniline as raw materials 1. Mix 1.8g (0.01mol) Add acetylsalicylic acid and 1.5ml (0.02mol) thionyl chloride into a 50ml reaction bottle and reflux until no bubbles are generated. Evaporate excess thionyl chloride to obtain a light yellow 2-acetoxybenzoyl chloride liquid. Add 5 ml of calcium chloride-dried acetone and set aside. 2. Add 1.4ml (0.01mol) triethylamine, 0.9g (0.01mol) aniline and 20ml dried acetone into a 1000ml reaction bottle, stir evenly, then slowly add 2-acetoxybenzene dropwise while cooling in an ice water bath. After the formyl solution is added dropwise, stir at room temperature for several hours. Evaporate the acetone under reduced pressure, add an appropriate amount of water, stir for a few minutes, let it stand, filter with suction, and wash with water. Hydrolyze the solid with 2 mol/L sodium hydroxide solution at room temperature for 4 hours, filter out the insoluble matter, and adjust the pH value of the filtrate to 4 with 3 mol/L hydrochloric acid. Filter with suction, wash with water, and recrystallize the solid with ethanol-water to obtain 2.2 g of white granular solid, with a yield of 80.6%. Aniline salicylate can also be produced by reacting methyl salicylate, phenyl salicylate and newly distilled aniline.  Acetylsalicylic acid and aniline are used as Raw materialsAdd 1.8g (0.01mol) acetylsalicylic acid and 1.5mL (0.02mol) sulfoxide dichloride into a 50mL reaction bottle and reflux until no bubbles are generated. Evaporate excess thionyl chloride to obtain a light yellow 2-acetoxybenzoyl chloride liquid. Add 5 mL of calcium chloride-dried acetone and set aside.
Acetylsalicylic acid and aniline are used as Raw materialsAdd 1.8g (0.01mol) acetylsalicylic acid and 1.5mL (0.02mol) sulfoxide dichloride into a 50mL reaction bottle and reflux until no bubbles are generated. Evaporate excess thionyl chloride to obtain a light yellow 2-acetoxybenzoyl chloride liquid. Add 5 mL of calcium chloride-dried acetone and set aside. 
Add 1.4mL (0..01) triethylamine, 0.9g (0.01mol) aniline and 20mL dried Acetone, stir evenly, then slowly add 2-acetoxybenzoyl chloride solution dropwise while cooling in an ice water bath, stir at room temperature for several hours after the dropwise addition. Evaporate the acetone under reduced pressure, add an appropriate amount of water, stir for a few minutes, let it stand, filter with suction, and wash with water. Hydrolyze the solid with 2 mol/L sodium hydroxide solution at room temperature for 4 hours, filter out the insoluble matter, and adjust the pH value of the filtrate to 4 with 3 mol/L hydrochloric acid. Filter with suction, wash with water, and recrystallize the solid with ethanol-water to obtain 2.2g of white granular solid, yield 80.6

Purpose
This product is an antifungal agent, mainly used to prevent mildew on cotton fabrics. Its sodium salt can be used as an antifungal agent for sheep rows, or polyvinyl chloride and other plastics, rubber, coatings, adhesives, leather and other materials. Pharmaceutical grade products are used to treat ringworm and for the preservation of pharmaceuticals. Salicylic acid anilide is added to 50% acetic acid, and bromine is added dropwise at 55°C for 1 hour to react to obtain 3,4′,5-tribromo salicylic acid anilide. This is an antiseptic used in soaps and cosmetics. Salicylanilide is also used as an intermediate for other fine chemicals.
