Sodium oleate
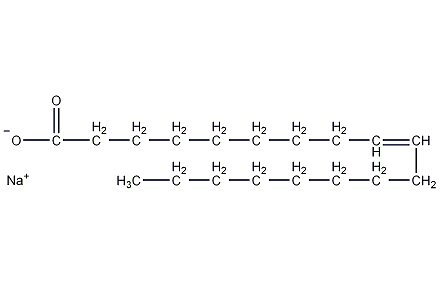
Structural formula
| Business number | 03VX |
|---|---|
| Molecular formula | C18H33NaO2 |
| Molecular weight | 304.44 |
| label |
(Z)-9-Octadenoic acid sodium salt, Sodium 9-octadecenoate, Sodium octadecene-[9]-acid, Cis-9-octadecenoic acid sodium salt, Oleic acid sodium salt, 9-Octadecenoic acid, sodiumsalt, Cooling and lubricating emulsified oil, synthetic fiber polish and lubricant, steel ball surface cleaner and anti-corrosion agent |
Numbering system
CAS number:143-19-1
MDL number:MFCD00004438
EINECS number:205-591-0
RTECS number:RK1200000
BRN number:None
PubChem number:24898044
Physical property data
1. Appearance: white powdery solid
2. Melting point (℃): 232-235
3. Solubility: soluble in water and easily soluble in ethanol.
Toxicological data
Acute toxicity: mouse intravenous LD50: 152 mg/kg; rabbit intravenous LDLo: 150 mg/kg. Slightly irritating to skin and mucous membranes.
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
None
Compute chemical data
1. Number of hydrogen bond donors: 0
2. Number of hydrogen bond acceptors: 2
3. Number of rotatable chemical bonds: 15
4. Topological molecular polar surface area (TPSA): 40.1
5. Number of heavy atoms: 21
6. Surface charge: 0
7. Complexity : 239
8. The number of isotope atoms: 0
9. The number of determined atomic stereocenters: 0
10. The number of uncertain atomic stereocenters: 0
11. Determine the number of stereocenters of chemical bonds: 1
12. Uncertain number of stereocenters of chemical bonds: 0
13. Number of covalent bond units :2
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: strong oxidizing agents. Soluble in water and ethanol.
Storage method
Packed in large iron drums, lined with plastic bags. Store in a cool, ventilated and dry place, away from moisture and sunlight.
Synthesis method
Dissolve 10g of oleic acid in 100mL of 95% ethanol, and then titrate with an ethanol solution of sodium hydroxide with a concentration of 0.5mol/L, using phenolphthalein as the indicator. After reaching the equivalence point, filter out the precipitated sodium oleate soap. If no precipitate occurs, the ethanol and water can also be evaporated to obtain a crude product, which can be recrystallized with an ethanol-ether mixed solvent.
Purpose
AvailableUsed as cooling lubricating emulsified oil for metal processing, polish and lubricant for synthetic fibers, steel ball surface cleaner and preservative. It is used as a flotation agent for ores, a waterproofing agent for fibers, and an emulsifier in oil-water systems.
