sodium propionate
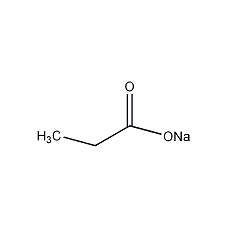
Structural formula
| Business number | 03RK |
|---|---|
| Molecular formula | C3H5NaO2 |
| Molecular weight | 96.06 |
| label |
propionic acid sodium salt, propionate sodium salt, preservative, antiseptic pesticides, food additives, Feed additives |
Numbering system
CAS number:137-40-6
MDL number:MFCD00002759
EINECS number:205-290-4
RTECS number:UF7525000
BRN number:3566934
PubChem number:24898252
Physical property data
1. Properties: Colorless transparent granular crystals or white crystalline powder, easily deliquescent.
2. Solubility: soluble in water and ethanol.
3. pH value of aqueous solution: 8.5-10.5
Toxicological data
Acute toxicity data:
Rat route unknown LD50: 8150mg/kg
Mouse oral LC50: 6332mg/kg
Mouse route unknown LC50: 7800mg/kg
Rabbit skin LD50: 1640mg/kg
Rabbit route unknown LD50: 6750mg/kg
Other multiple dose data:
Rat route unknown TDLo: 24817mg/kg/9W-I is irritating
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 40.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 44.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure
Storage method
Sealed and dry storage
Synthesis method
1. Mainly adopt synthetic method. Propionic acid and sodium hydroxide are used as raw materials for synthesis reaction, and then dehydrated, refined and dried to obtain
2.Dissolve sodium carbonate in hot water, slowly add propionic acid at 70°C, then heat to boiling, control the ph value at 6.8~7.3, decolorize and filter , concentrate under reduced pressure, cool, filter and dry to get the finished product. The reaction formula is as follows:
![]()
3. Neutralized by propionic acid and sodium hydroxide, concentrated, crystallized, separated and dried And get.
4.Obtained from the neutralization of propionic acid and sodium hydroxide or sodium carbonate .
Purpose
1. Cosmetic preservatives. It has a wide range of antibacterial effects on molds, yeasts and bacteria. The antibacterial effect is particularly obvious in acidic solutes. The amount added in cosmetics is usually no more than 2%. Pesticides, fungicides. Determination of aminotransferases. It is used as a masking agent in tanning to improve the alkali resistance of the leather and the uniformity of tanning. 2.Sodium propionate is an acid-type food preservative, and its antibacterial effect is affected by the pH value of the environment. It has a strong inhibitory effect on various types of molds, Gram-negative bacilli or aerobic bacilli in acidic media. It is particularly effective in preventing the production of aflatoxin and is almost ineffective against yeast. In the food industry, it can be used to preserve pastries, and the usage amount is 2.5g/kg (calculated as propionic acid). 3.Sodium propionate is a preservative and has a strong inhibitory effect on various types of molds, aerobic bacilli or gram-negative bacilli in acidic media. . It is particularly effective in preventing the production of aflatoxin, but has almost no effect on yeast. It can also be used as a food preservative. 4.Used as a masking agent in tanning to improve the alkali resistance of the leather and the uniformity of tanning.
