1-Decanethiol
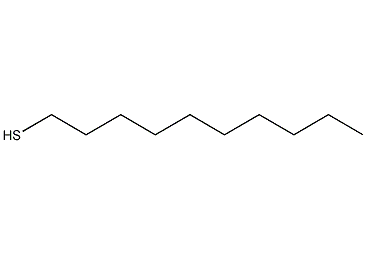

Structural formula
| Business number | 03VS |
|---|---|
| Molecular formula | C10H22S |
| Molecular weight | 174.35 |
| label |
Decanethiol, n-decanethiol, 1-Thiodecane, n-Dodecyl mercaptan, CH3(CH2)9SH, Sulfur compound solvents, Rubber intermediates |
Numbering system
CAS number:143-10-2
MDL number:MFCD00004884
EINECS number:205-584-2
RTECS number:None
BRN number:1735223
PubChem number:24893492
Physical property data
1. Properties: colorless or light yellow liquid. Has a special odor.
2. Relative density (g/mL, 20/4ºC): 0.847
3. Refractive index (20ºC): 1.4565
4. Flash point ( ºC): 98
5. Melting point (ºC): -26
6. Boiling point (ºC, 1.733kpa): 113~114
7. Dissolution Properties: Miscible with ethanol and ether, slightly soluble in water.
Toxicological data
Irritating to eyes, respiratory system and skin.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 56.27
2. Molar volume (cm3/mol): 207.6
3. Isotonic specific volume (90.2K): 481.8
4. Surface tension (dyne/cm): 28.9
5. Polarizability (10-24cm3): 22.30
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 61.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. Protective clothing should be worn when using.
Storage method
Store sealed in a cool, dry place.
Synthesis method
None
Purpose
Used as solvents, organic synthesis intermediates, and synthetic rubber intermediates.