1-Methoxy-2-propanol 1-Methoxy-2-propanol
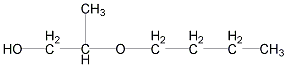
Structural formula
| Business number | 02W6 |
|---|---|
| Molecular formula | C4H10O2 |
| Molecular weight | 90 |
| label |
Propylene glycol monomethyl ether, 1,2-Propanediol-1-monomethyl ether, 2-hydroxypropyl methyl ether, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:107-98-2
MDL number:MFCD00004537
EINECS number:203-539-1
RTECS number:UB7700000
BRN number:1731270
PubChem number:24884249
Physical property data
1. Properties: Colorless transparent liquid
2. Density (g/mL, 20/4℃): 0.922
3. Relative vapor density (g/mL, Air = 1): 3.12
4. Melting point (ºC, pouring point): -97
5. Boiling point (ºC, normal pressure): 118
6. Refractive index (20ºC): 1.4034
7. Viscosity (mPa·s, 25ºC): 1.75
8. Flash point (ºC, opening): 39
9. Heat of evaporation (KJ/mol): 40.6
10. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.56
11. Steam Pressure (kPa, 2ºC): 1.01
12. Vapor pressure (kPa, 21.7ºC): 1.33
13. Solubility: miscible with water. Can dissolve grease, rubber, natural resin, ethyl cellulose, nitrocellulose, polyvinyl acetate, polyvinyl butyral, alkyd resin, phenolic resin, urea-formaldehyde resin, etc.
14. Relative density (25℃, 4℃): 0.919
15. Refractive index at room temperature (n25): 1.4017
Toxicological data
It is slightly toxic, with an oral LD50 of 6.6g/kg in rats. There is no obvious irritation to the skin, but toxic doses can be absorbed through the skin. The main symptoms of animal poisoning are suppression and incomplete anesthesia. When rats were exposed to a vapor concentration of 40.18g/m3 for 5 to 6 hours, half of them died.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 23.81
2. Molar volume (cm3/mol): 98.8
3. Isotonic specific volume (90.2K ): 225.0
4. Surface tension (dyne/cm): 26.9
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 9.44
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 6
8.�Surface charge: 0
9. Complexity: 28.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters :0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with moisture.
2.The oral LD of mice is 506.6g/kg. The vapor has an irritating effect on the eyes and nasal mucosa of animals. At the saturated concentration (18.4~36.8mg/L), it can cause death within a few hours. Liquid contact with the skin (rabbits) can cause anesthesia, and long-term or large-dose (>10mL/kg) exposure can be fatal (LD5013~14g/kg). Protective equipment should be worn during operation. In addition, the generated vapor should be removed on site to protect the skin.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire, heat and water sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. This product is a flammable liquid and should be handled as such. Storage tanks and reactors should be covered with dry nitrogen. Electrical equipment should be explosion-proof. Store and transport according to regulations for flammable materials.
Synthesis method
It is produced by reacting 1,2-propylene oxide and methanol in the presence of a catalyst, and then crude distilling and rectifying the reactants.
![]()
Purpose
Mainly used as solvent, dispersant and diluent, also used as fuel antifreeze, extraction agent, etc. Used as a solvent for nitrocellulose, a compounding agent for brake oil and detergents, etc.
Widely used in coatings and cleaners. Specific applications: Reactive solvent for water-based coatings; Reactive solvent and coupling agent for solvent-based printing inks; Solvent for ballpoint pens and fountain pens; Coupling agent and solvent for household and industrial cleaners, rust removers and hard surface cleaners; Agriculture Solvent for insecticides; used in glass cleaner formulations when mixed with propylene glycol n-butyl ether.